

Background: EXCEED (NCT02745080) was the first fully blinded head-to-head trial to evaluate the efficacy and safety of secukinumab (SEC) versus (vs) adalimumab (ADA) monotherapy in patients with active psoriatic arthritis (PsA) with a primary endpoint of American College of Rheumatology (ACR) 20 at Week 52. Although SEC narrowly missed statistical significance for superiority vs ADA, numerically higher response for other musculoskeletal endpoints and composite indices were observed with SEC. 1
Objectives: To explore the effect of SEC and ADA on ACR core components, function and Health-related Quality of Life (HRQoL) outcomes.
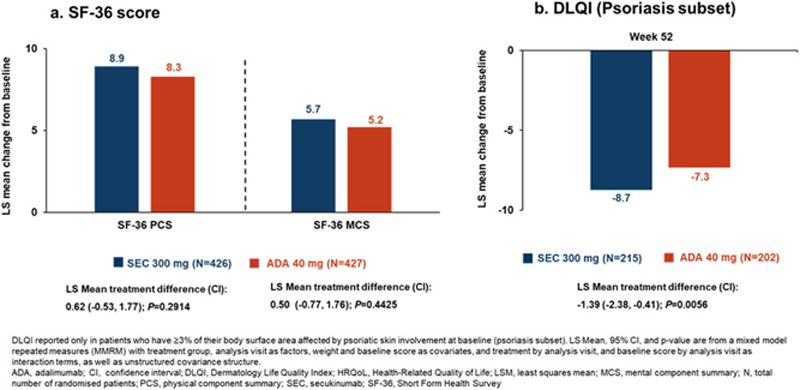
Methods: Patients were randomised 1:1 to receive SEC 300 mg (N=426) subcutaneous (s.c.) at baseline, Week 1-4, followed by every 4 weeks until Week 48 or ADA 40 mg (N=427) s.c. at baseline followed by same dosing every 2 weeks until Week 50. The primary, key secondary and some exploratory endpoints at Week 52 were previously reported. 1 A supportive analysis for ACR50 response using logistic regression model and trimmed means model for Health Assessment Questionnaire-Disability Index (HAQ-DI) with gender and smoking status as factors was performed to adjust for imbalances in baseline characteristics. An exploratory analysis of ACR core components with SEC vs ADA at Week 52 was conducted using a mixed-effects repeated measures model that included tender and swollen joint counts, patient and physician global assessment, PsA pain (VAS) and erythrocyte sedimentation rate. HRQoL variables were also exploratory and assessed based on Short Form Health Survey Physical/Mental Component Summary (SF-36 PCS/MCS) scores and Dermatology Life Quality Index (DLQI).
Results: The demographic and baseline disease characteristics were comparable across treatment groups, except for an imbalance in sex (females: 51.2% vs 46.4%) and smoking status (yes: 21.8% vs 17.8%) in SEC and ADA group, respectively. At Week 52, ACR50 responses were 49.0% and 44.8% (
P
=0.0929) and HAQ-DI mean change from baseline were −0.69 and −0.58 (
P
=0.0314) in SEC and ADA treatment groups, respectively after adjusting for gender and smoking status. No major difference across ACR core components was observed in both treatment groups at Week 52 (
Conclusion: Secukinumab provided similar improvements in ACR core components and SF-36 based quality of life at Week 52 with adalimumab. Greater improvement in HAQ-DI response and DLQI was demonstrated with secukinumab compared to adalimumab.
REFERENCES:
[1]McInnes IB, et al. Lancet . 2020; 395:1496–505.
ACR Core Components at Week 52
| Variables |
Secukinumab 300 mg
|
Adalimumab 40 mg
| P -value | ||
| BL, mean ± SE | LSM change from BL ± SE | BL, mean ± SE | LSM change from BL ± SE | ||
| Tender joint score
| 19.4 ± 13.86 | −14.27 ± 0.44 | 20.6 ± 14.81 | −13.90 ± 0.45 | 0.5549 |
| Swollen joint score
| 9.7 ± 7.30 | −8.41 ± 0.19 | 10.2 ± 7.86 | −8.06 ± 0.20 | 0.1962 |
| Patients global assessment | 64.0 ± 19.67 | −33.81 ± 1.14 | 61.9 ± 20.75 | −31.61 ± 1.19 | 0.1825 |
| Physicians global assessment | 60.0 ± 17.12 | −46.24 ± 0.80 | 61.4 ± 15.92 | −43.63 ± 0.84 | 0.0243 |
| Psoriatic arthritis pain (VAS) | 58.6 ± 23.49 | −30.21 ± 1.18 | 57.9 ± 22.42 | −29.44 ± 1.23 | 0.6500 |
| Erythrocyte sedimentation rate (mm/h) | 23.8 ± 18.93 | −9.63 ± 0.62 | 23.9 ± 17.99 | −9.28 ± 0.64 | 0.7029 |
LS mean and nominal P -values are from a mixed-effects repeated measures model with treatment group, analysis visit as factors, weight and BL score as covariates, and by treatment and BL score as interaction terms, unstructured covariance structure. ACR, American College of Rheumatology; BL, baseline; LSM, least squares mean; N, total number of randomised patients; SE, standard error; VAS, visual analogue scale
HRQoL Analysis at Week 52
Disclosure of Interests: Philippe Goupille Speakers bureau: AbbVie, Amgen, Biogen, BMS, Celgene, Chugai, Janssen, Eli Lilly, Medac, MSD, Nordic Pharma, Novartis, Pfizer, Sanofi and UCB, Consultant of: AbbVie, Amgen, Biogen, BMS, Celgene, Chugai, Janssen, Eli Lilly, Medac, MSD, Nordic Pharma, Novartis, Pfizer, Sanofi and UCB, Grant/research support from: AbbVie, Amgen, Biogen, BMS, Celgene, Chugai, Janssen, Eli Lilly, Medac, MSD, Nordic Pharma, Novartis, Pfizer, Sanofi and UCB, Frank Behrens Paid instructor for: Eli Lilly, Consultant of: Pfizer, AbbVie, Sanofi, Eli Lilly, Novartis, Genzyme, Boehringer Ingelheim, Janssen, MSD, Celgene, Roche and Chugai, Grant/research support from: Pfizer, Janssen, Chugai, Celgene and Roche, Laura C Coates Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Biogen, BMS, Celgene, Domain, Eli Lilly, Gilead, GSK, Janssen, Medac, Novartis, Pfizer, Serac and UCB, Grant/research support from: AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB, Jordi Gratacos-Masmitja Speakers bureau: AbbVie, Amgen, BMS, Celgene, Janssen, Eli Lilly, Novartis and Pfizer, Consultant of: AbbVie, Amgen, BMS, Celgene, Janssen, Eli Lilly, Novartis and Pfizer, Grant/research support from: AbbVie, Amgen, BMS, Celgene, Janssen, Eli Lilly, Novartis and Pfizer, Philip J Mease Speakers bureau: AbbVie, Amgen, Genentech, Janssen, Eli Lilly, Merck, Novartis, Pfizer, and UCB, Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Galapagos, Celgene, Genentech, Gilead, Janssen, Eli Lilly, Novartis, Pfizer, SUN Pharma, and UCB, Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Galapagos, Genentech, Gilead, Janssen, Eli Lilly, Merck, Novartis, Pfizer, SUN Pharma, and UCB, Dafna D Gladman Consultant of: Amgen, AbbVie, BMS, Celgene, Eli Lilly, Gilead, Galapagos, Janssen, Novartis, Pfizer and UCB, Grant/research support from: Amgen, AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB, Peter Nash Speakers bureau: Novartis, Abbvie, Roche, Pfizer, BMS, Janssen, Celgene, UCB, Eli Lilly, MSD, Sanofi, Gilead, Consultant of: Novartis, Abbvie, Roche, Pfizer, BMS, Janssen, Celgene, UCB, Eli Lilly, MSD, Sanofi, Gilead, Grant/research support from: Novartis, Abbvie, Roche, Pfizer, BMS, Janssen, Celgene, UCB, Eli Lilly, MSD, Sanofi, Gilead, Arthur Kavanaugh Consultant of: AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, and UCB, Grant/research support from: AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, and UCB, Ruvie Martin Shareholder of: Novartis, Employee of: Novartis, Weibin Bao Shareholder of: Novartis, Employee of: Novartis, Corine Gaillez Shareholder of: Novartis and BMS, Employee of: Novartis, Iain McInnes Speakers bureau: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Eli Lilly, Novartis, Pfizer, and UCB, Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Eli Lilly, Novartis, Pfizer, and UCB, Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Eli Lilly, Novartis, Pfizer, and UCB.