

Background: Up to 80% of patients with psoriatic arthritis (PsA) have comorbid psoriasis (PsO). 1 However, data on the impact of comorbid PsA on treatment persistence in PsO patients are limited with mixed findings. 2–4 Other factors have been implicated in predicting response to treatment, including obesity, which is associated with PsA and has demonstrated a varying effect on biologic persistence. 2,3,5,6
Objectives: The primary objective of this study was to evaluate whether comorbid PsA and obesity impact ustekinumab (UST) persistence in PsO patients using the British Association of Dermatologists Biologic and Immunomodulators Register (BADBIR). Additionally, the effect of line of therapy was investigated.
Methods: This retrospective observational study used data from PsO patients receiving UST in the BADBIR (first enrolled 07/2009; 10/2020 data cut-off). Time to discontinuation (TTD) was defined as time from treatment start date until treatment stop date, censoring for loss to follow-up where patients are lost to follow-up or active in the registry without a recorded treatment stop date. Kaplan–Meier survival analysis was used to estimate probability of discontinuation over time and median TTD. Patients were stratified by PsA diagnosis (recorded as a comorbidity at enrolment) and prior biologic experience (history of treatment with ≥1 other biologic). The log-rank test was used to compare persistence among groups. Hazard ratios (HRs), confidence intervals (CI) and p-values for effect of obesity, PsA status and prior biologic exposure on TTD were generated using Cox proportional hazards regression.
Results: Univariate analysis found that median TTD was shorter for patients with comorbid PsO and PsA (5.04 years; n=585) vs. patients with PsO alone (7.19 years; n=2698; p<0.0001). Biologic-experienced patients with comorbid PsO and PsA (n=292) had a significantly higher likelihood of discontinuation than any other group, with a median TTD of 3.84 years (p<0.0001;
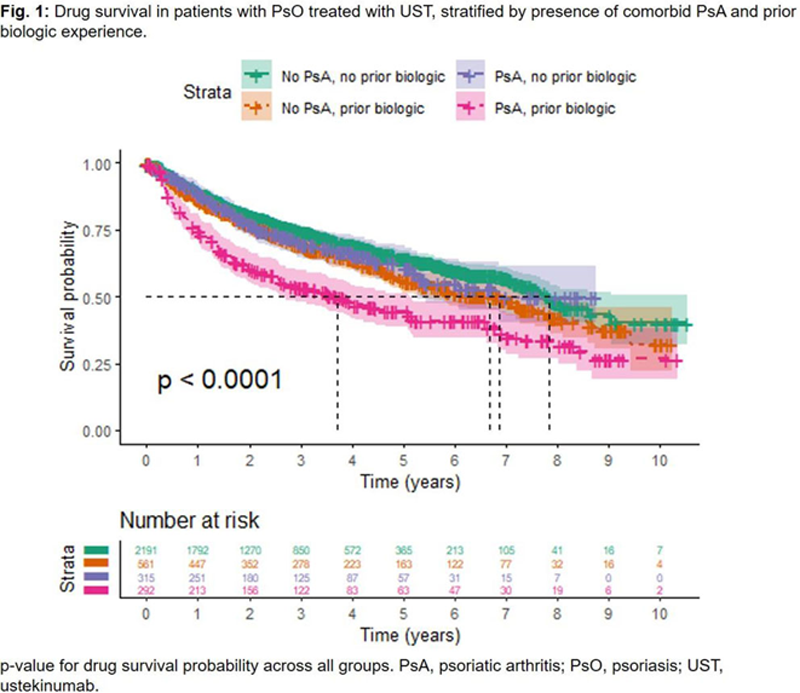
Conclusion: Comorbid PsA was associated with reduced time-to-discontinuation of UST in biologic-experienced patients, but not biologic-naïve patients. Obesity also impacted TTD but independently of PsA status.
REFERENCES:
[1]Coates and Helliwell. Clin Med 2017;17:65–70
[2]Menter et al. J Eur Acad Dermatol Venereol 2016;30:1148–58
[3]Mourad et al. Br J Dermatol 2019;181:450–8
[4]Yiu et al. Br J Dermatol 2020;183:294–302
[5]Mease et al. RMD Open 2015;1:e000181
[6]Costa et al. Clin Rheumatol 2019;38:2355–62
Multivariate analysis of UST discontinuation in PsO patients (N=3,174).
| Covariate | Status (n ) | HR | 95% CI | p-value |
| Comorbid PsA | (n=2609)
| ref | - | - |
| (n=565)
| 1.12 | 0.90–1.40 | 0.31 | |
| Biologic experience | No prior biologic (n=2371) | ref | - | - |
| Prior biologic (n=803) | 1.21 | 1.04–1.43 | 0.017 | |
| PsA*prior biologic experience | (n=539)
| ref | - | - |
| PsA, prior biologic
| 1.45 | 1.06–1.97 | 0.018 | |
| Obesity (BMI, kg/m 2 ) | (n=1554)
| ref | - | - |
| (n=1620)
| 1.15 | 1.02–1.31 | 0.027 |
BMI, body mass index; CI, confidence interval; HR, hazard ratio; PsA, psoriatic arthritis; PsO, psoriasis; ref, reference; UST, ustekinumab.
Disclosure of Interests: Alexis Ogdie Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Janssen, Eli Lilly, Novartis and Pfizer, Grant/research support from: Pfizer to Penn, Novartis to Penn, Amgen to Forward/NDB, William Tillett Speakers bureau: AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB, Consultant of: AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, MSD, Pfizer and UCB, Grant/research support from: AbbVie, Celgene, Eli Lilly, Janssen and UCB, Alun Passey Employee of: Janssen-Cilag Ltd, Patricia Gorecki Employee of: Janssen-Cilag Ltd.