

Background: AR882 is a novel, potent and selective uric acid transporter 1 (URAT1) inhibitor in Phase 2 development for the treatment of hyperuricemia and gout. In Phase 1 healthy subject studies, AR882 demonstrated good dose proportionality, a long effective half-life and sustained serum urate (sUA) lowering effects, consequently making it suitable for once-daily dosing. In addition, AR882 was well tolerated following single and multiple dosing.
Objectives: This Phase 2a study evaluated the pharmacokinetics (PK), pharmacodynamics (PD), and safety of AR882 following monotherapy in patients with gout. PK and sUA lowering effects in patients with renal impairment and normal renal function were also compared.
Methods: A total of 30 adults with gout (sUA >7 mg/dL) were enrolled and 28 had post-baseline PKPD assessments performed. AR882 was administered at escalating doses of 25, 50 and 75 mg, with each dose administered once daily for 7 days. Serial blood samples were collected to measure AR882 PK and sUA levels at the end of each treatment week. Urine samples were collected for assessment of uric acid excretion. Laboratory safety tests, vital signs, and electrocardiograms were collected throughout the study.
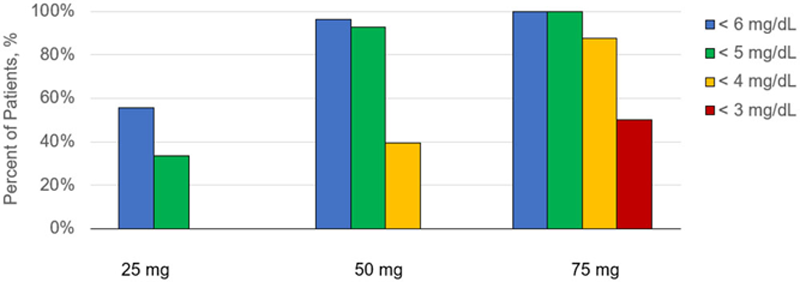
Results: Following once daily administration in patients with gout, AR882 exposures increased dose proportionally between 25 and 75 mg. Mean sUA levels were maximally reduced from baseline (mean 8.9 mg/dL) to 5.6, 4.2 and 3.2 mg/dL at the 25-, 50- and 75-mg dose levels, respectively. The corresponding percent reductions were 36.8%, 52.7% and 61.5%. The effects were sustained throughout the entire dosing day with minimal fluctuation. In patients receiving 25 mg AR882 (N=9), 56% achieved sUA levels below 6 mg/dL. At 50 mg (N=28), 96% of patients had sUA levels below 6 mg/dL and 93% were below 5 mg/dL. At the 75 mg dose (N=8), all patients (100%) achieved levels below 5 mg/dL, and 88% were below 4 mg/dL. Among these 28 gout patients, 17 patients had normal renal function (CrCL>90 mL/min) and 11 patients had mild impairment (CrCL 60-90 mL/min). AR882 exposures were similar between patients with mild renal impairment and those with normal renal function. Both groups showed nearly identical sUA lowering effects following each 7-day dosing period. Response rates were also similar between these groups, including at the 50 and 75 mg dose levels. Consistent with its sUA lowering effects, dose-dependent increases in fractional excretion of uric acid were observed across all doses.
AR882 was well tolerated at all doses tested. All AEs were mild or moderate in severity and most were considered not related to study treatment. There were no serious AEs or AEs resulting in study drug discontinuation. There were no clinically significant laboratory or ECG abnormalities noted.
Conclusion: In patients with gout, AR882 exhibited dose proportional increases in plasma exposure and dose-dependent reductions in sUA. Nearly all patients had sUA levels below 5 mg/dL at doses ≥50 mg, and about 90% of patients had sUA levels <4 mg/dL at 75 mg dosing. Similar PK exposures and sUA lowering effects were observed in patients with mild renal impairment compared to those with normal renal function. Current data suggest that AR882 50 mg may offer improved efficacy over existing standard-of-care for gout and AR882 75 mg may have utility in the treatment of patients with severe or refractory gout disease.
Percent of Patients with sUA below 6 mg/dL, 5 mg/dL, 4 mg/dL or 3 mg/dL Following Once-Daily Oral Doses of AR882.
Mean (SD) sUA Levels Following Once-Daily Oral Doses of AR882.
| Treatment | N | Time (hr), sUA (mg/dL) | |||
| 0 | 6 | 12 | 24 | ||
| Baseline | 30 | 8.8 (1.1) | 8.8 (1.1) | 9.0 (1.0) | 8.9 (1.1) |
| 25 mg | 9 | 6.1 (0.4) | 5.6 (0.4) | 5.6 (0.3) | 5.8 (0.4) |
| 50 mg | 28 | 4.7 (0.9) | 4.2 (0.9) | 4.3 (0.8) | 4.7 (1.0) |
| 75 mg | 8 | 3.7 (1.1) | 3.2 (0.9) | 3.2 (0.8) | 3.6 (1.0) |
| 50 mg (normal renal function) | 17 | 4.8 (1.1) | 4.3 (1.1) | 4.4 (0.9) | 4.8 (1.2) |
| 50 mg (mild impairment) | 11 | 4.5 (0.6) | 4.1 (0.6) | 4.3 (0.5) | 4.6 (0.6) |
Disclosure of Interests: Zancong Shen Employee of: Arthrosi Therapeutics Inc, Elizabeth Polvent Employee of: Arthrosi Therapeutics Inc, Vijay Hingorani Consultant of: Arthrosi Therapeutics Inc, Andrea Clouser Roche Employee of: Arthrosi Therapeutics Inc, Chris Colton Employee of: Arthrosi Therapeutics Inc, Rongzi Yan Employee of: Arthrosi Therapeutics Inc, Shunqi Yan Employee of: Arthrosi Therapeutics Inc, Li-Tain Yeh Employee of: Arthrosi Therapeutics Inc.