

Background: Latent tuberculous infection (LTBI) is very common in the world and screening for it is essential before starting treatment with biotechnological drugs
Objectives: The aims of our study were to assess the prevalence in Apulia of LTBI among patients affected with rheumatic disease and to record the cases of tuberculosis (TB) infection among patients treated with biologic agents.
Methods: We analysed data of patients included in BIOPURE registry from 2008 to 2018, who underwent Quantiferon (QTF) test as routinely screening for biologic treatment. Demographic and clinical data were recorded at the time of the first QTF assessment and this time point was considered the “baseline” of the study. Data regarding further QTF tests performed during follow-up was also acquired by electronic charts. Prophylaxis administration and bDMARD treatments were recorded for patients with positive QTF test. All tuberculosis infections were recorded during the entire time of follow-up.
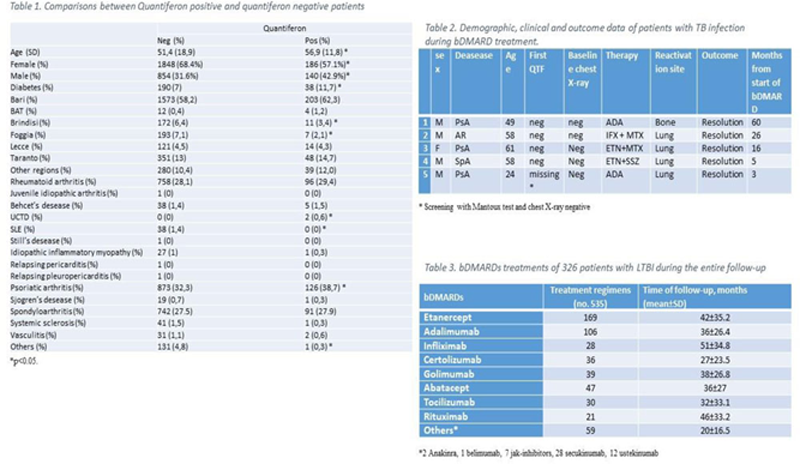
Results: Three thousand thirty-five patients (female 67.2%, mean age 52 ± 18.3 years) were included in these study, 2692 patients (88.7%) had inflammatory arthritis (28.2% rheumatoid arthritis, 33% psoriatic arthritis and 27.4% spondyloarthritis), 129 (4.2%) patients had connective tissue disease, whereas 214 (7.1%) patients were affected by others rheumatic diseases. The prevalence of LTBI was 10.7% (326 patients) at baseline. Comparisons between positive and negative patients for QTF are reported in
Conclusion: Our study shows a prevalence of LTBI of 10.7% in Apulian patients affected with rheumatic disease. bDMARDs therapy appears to be safe in patients with positive QTF test treated according to current recommendations 1 . However, cases of primary TB infections, especially in patients receiving anti-TNFα drugs, have been observed.
REFERENCES:
[1]Cantini F, et al, Guidance for the management of patients with latent tuberculosis infection requiring biologic therapy in rheumatology and dermatology clinical practice, Autoimmun Rev (2015).
Disclosure of Interests: None declared