

Background: Cardiovascular disease (CVD) risk in patients with chronic inflammatory arthritis (IA), including rheumatoid arthritis (RA), psoriatic arthritis (PsA) and ankylosing spondylitis (AS), is substantially increased compared to the general population. New evidence strengthens the notion that the excess risk of CVD morbidity and mortality in patients with IA is related to both traditional (e.g. hypertension, diabetes, smoking) and novel CVD risk factors, including chronic inflammation, leading to an accelerated atherosclerosis. How to minimize such increased CVD prevalence is still poorly understood, and whether more intensive traditional risk factor control or disease specific risk factor should be targeted is still matter of debate.
Objectives: The aim of this systematic review was to identify intervention targeting CVD or inflammatory arthritis associated with improvement of CV risk outcomes (estimated CV risk, CV events, endothelial function, arterial stiffness, subclinical atherosclerosis) in adult patients with diagnosis of inflammatory arthritis (RA, PsA and AS).
Methods: Two independent reviewers retrieved randomized controlled trials of interest from systematic searches of Medline, Embase and Cochrane database (20th April 2020). Data extraction was performed using standard template; the quality of each included trials was assessed with the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [1]. Systematic review was conducted following the Preferred Reporting Items for systematic reviews and Meta-analysis (PRISMA) statement.
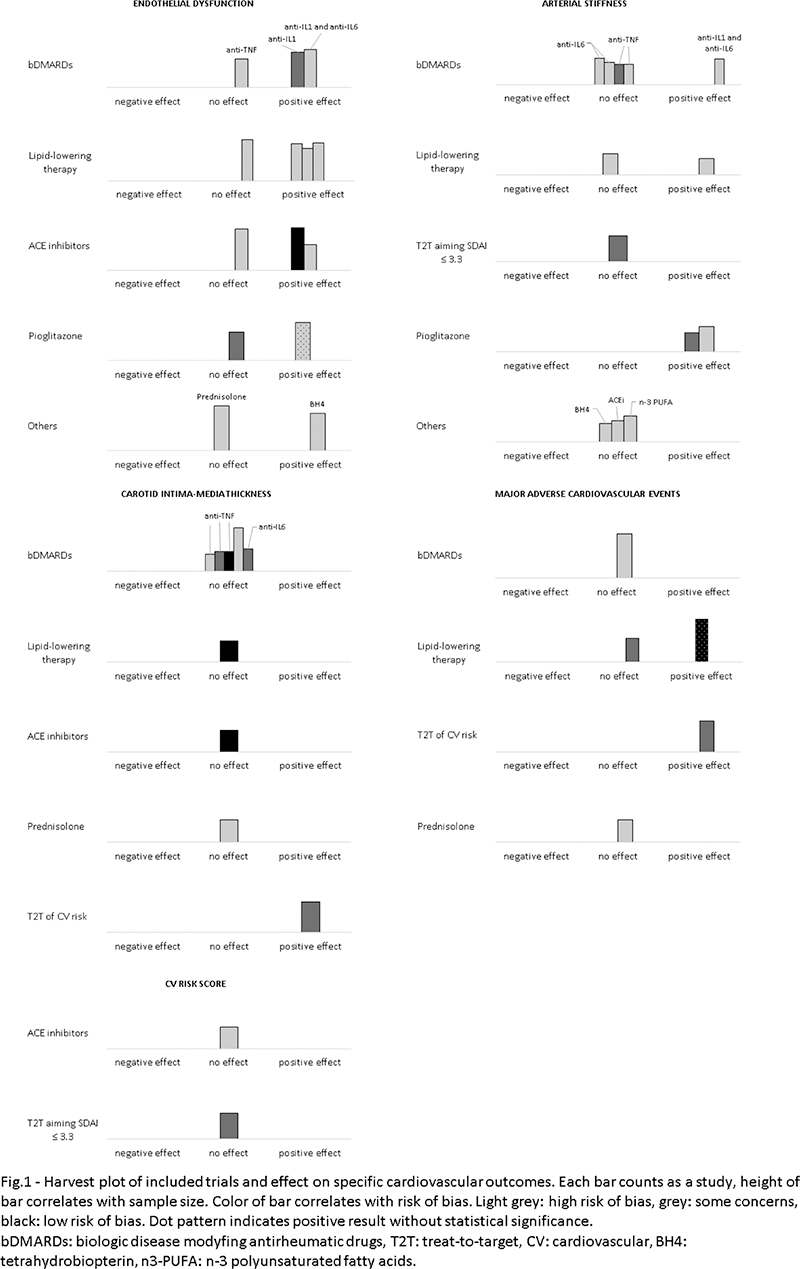
Results: Out of total of 4823 articles, 27 met the inclusion criteria. Among these, most (n=22) involved RA patients, one trial was based on mixed IAs patients and the remaining (n=4) were performed on spondylarthritis population. Total number of patients was 8045. Overall risk of bias was high in most of per protocol analysis trials (90%) and in 26.7% of intention-to-treat analysis trials. Four trials evaluated major adverse cardiovascular events (MACE) incidence and one of these demonstrated a significant reduction in incidence of MACE in RA patients underwent a treat-to-target strategy of CV risk factor. The same study also demonstrated a significant reduction in progression of subclinical atherosclerosis (carotid Intima-media thickness, cIMT), while other trials (n= 8) exploring effect of rosuvastatin, enalapril, tocilizumab and TNF-inhibitors failed to reach a similar result. Endothelial dysfunction, predominantly measured as reduce flow mediated dilatation (FMD), was widely used as surrogate outcome of CVD and it appeared to be significantly improved by treatment with statins, ACE-inhibitors, anakinra and tocilizumab. Treatment with pioglitazone, anakinra or tocilizumab in three trials significantly ameliorated arterial stiffness, estimated with pulse wave velocity (PWV), Cardio-ankle vascular index (CAVI) or augmentation index (AI). Two studies explored how a reduction of estimated CV risk could be achieved after treatment with enalapril and tight-control strategy aiming to SDAI ≤ 3.3. Results of both trials didn’t demonstrate any variation in QRISK3-2018 and Framingham risk score, respectively.
Conclusion: Optimal CVD management in IA patients remains undefined and it should be implemented as stated in international guidelines. Randomized controlled trials exploring efficacy of prevention strategy are few and predominantly focused on surrogate outcome measures of cardiovascular risk.
REFERENCES:
[1]Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898.
Disclosure of Interests: Carlo Garaffoni: None declared, Antonio Marangoni: None declared, Marcello Govoni Speakers bureau: Abbvie, Pfizer, Galapagos, BMS, Eli-Lilly, Paid instructor for: Pfizer, Consultant of: Abbvie, BMS, Novartis, Astrazeneca, Pfizer, Carlo Alberto Scirè Grant/research support from: AbbVie, Lilly