

Background: AntiTNF-α biosimilars are broadly available for the treatment of inflammatory arthritis. There are a lot of data concerning the maintenance of clinical efficacy after switching from originators to biosimilars. However, there is lack of data on the switch between biosimilars. The current evidence on the safety, efficacy, and immunogenicity of switching multiple times from a biosimilar to another biosimilar comes from a limited number of randomized-controlled trials and real world evidence studies.
Objectives: The aim of our work was to evaluate the disease activity trend after multiple switching from ADA originator-Humira (oADA) to its biosimilars (bsADA; ABP 501 and SB5 subsequently) in a cohort of inflammatory arthritis patients.
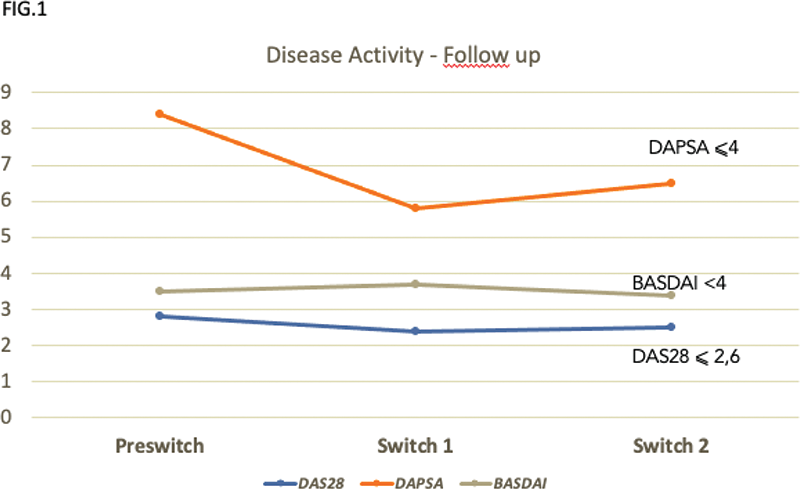
Methods: In this real-life study, we selected patients with clinical diagnosis of rheumatoid Arthritis (RA), psoriatic Arthritis (PsA) and ankylosing spondylitis (AS). Patients had been previously treated with oADA and switched to the bsADA (first ABP 501 and then SB5). At each outpatient visit, we recorded demographic features (age, sex, and time since diagnosis) and the following disease activity measures: DAS28, DAPSA, BASDAI and the HAQ. Rheumatoid factor (RF), anti-citrullinated protein antibody (ACPA), C-reactive protein (CRP) and HLAB27 were also measured over the observational period (visits 0, 12, 24 and 36 months). The disease activity was evaluated during the year before the introduction of the bsADA, and then evaluated in the following 36 months during the first and the second bsADA treatment. We also examined whether some baseline characteristics, such as the duration of ADA treatment, concomitant therapy, comorbid disease and baseline disease activity, could influence the bsADA discontinuation.
Results: We evaluated the 3-year drug survival and efficacy of the multiple switch of bsADA in RA, PsA and AS patients, previously treated with oADA in 127 patients (
Baseline characteristics of RA, PsA and AS patients
| Characteristics | Total cohort N=127 | RA N=41 | PsA N=52 | AS N=34 |
|---|---|---|---|---|
| Female gender (%) | 75 (60) | 35 (85) | 31 (59) | 9 (26) |
| Age (years) | 61.7±12.78 | 60.5±12.64 | 61.55±11.6 | 56.3±10.71 |
| Disease duration (years) | 15.86±9.49 | 17.23±10.64 | 13.75±6.57 | 14.92±9.50 |
| Comorbidities, (%) | 17,8 (14) | 5,7 (14) | 9,8 (19) | 2 (6) |
| ACPA+, n (%) | - | 25 (63.3) | - | - |
| RF+, n (%) | 24 (62.5) | |||
| HLAB27+, n (%) | - | - | 8 (15) | 17 (50) |
| CRP (mg/L) | 1.65±2.27 | 2.31±1.90 | 0.42±0.27 | 1.36±1.91 |
| DAS 28 | - | 2.65±0.75 | ||
| DAPSA | - | 8.25±3.69 | ||
| BASDAI | - | 3.23±1.58 | ||
| HAQ | 0.73±0.57 | 0.72±0.55 | 0.73±0.52 | 0.76±0.69 |
| Prednisone, n (%) | 46 (36) | 23 (55) | 20 (44) | 3 (13) |
| Combo Therapy | 65 (51) | 29 (68) | 25 (48) | 11 (32) |
| oADA duration (months) | 49.74±40.75 | 56.43±41.27 | 25.00±13.67 | 40.08±37.78 |
| ABP501 duration (months) | 12.42±2.41 | 12.77±2.34 | 12.82±1.49 | 12.49±2.95 |
| SB501 duration (months) | 11.92±1.41 | 12.47±1.74 | 12.72±1.49 | 12.41±1.95 |
ABP501 (Adalimumab biosimilar, Amgevita), SB501 (Adalimumab biosimilar, Imraldi)
Conclusion: No difference was found between oADA and bsADA in terms of efficacy. This real-life study confirms the similar efficacy profile of multiple switch bsADA with long-term retention and a good safety profile in inflammatory arthritis patients.
REFERENCES:
[1]Feagan BG et al. Adv Ther. 2020 Nov;37(11):4491-4518. doi: 10.1007/s12325-020-01472-1. Epub 2020 Sep 10.
Disclosure of Interests: None declared