

Background: T-cell priming and T-cell-dependent B-cell responses require an intact cluster of differentiation (CD)40/CD40L pathway. CD40 is expressed on the surface of B-cells, dendritic cells, antigen-presenting cells, and non-immune cell types; its ligand, CD40L (CD154), is expressed on the surface of activated T-cells, platelets, and other cell types. Blockade of CD40/CD40L interaction has been shown to ablate primary and secondary T-cell dependent antibody response (TDAR).
Objectives: We hypothesized that KPL-404, an anti-CD40 monoclonal antibody which inhibits interaction between CD40 and CD40L, would block T-cell dependent, B-cell-mediated autoimmunity in this Phase 1 study in healthy participants.
Methods: This randomized, double-blind, placebo-controlled, first-in-human study of KPL-404 in healthy participants was designed with two single-ascending-dose arms: single intravenous (IV) doses of 0.03 mg/kg, 0.3 mg/kg, 1 mg/kg, 3 mg/kg, or 10 mg/kg and single subcutaneous (SC) doses of 1 mg/kg or 5 mg/kg. The primary objective was safety and tolerability of KPL-404; secondary and exploratory objectives included pharmacokinetic (PK) parameters, TDAR inhibition, and receptor occupancy (RO). To evaluate TDAR inhibition, participants post-KPL-404 administration were immunized with 1 mg intramuscular injection of the test antigen Keyhole Limpet Hemocyanin (KLH) on day 4 and day 29 to elicit a primary and secondary Immunoglobulin (Ig) response, respectively. To evaluate RO, free and total CD40 receptor levels (percent change from baseline) on B-cells (whole blood) were measured using flow cytometry.
Results: There were no dose-limiting or dose-related safety findings in healthy participants after KPL-404 administration. One unrelated serious adverse event (patella fracture following a fall) occurred in the 10 mg/kg IV arm. The PK profile of KPL-404 in serum after IV or SC administration had low to moderate variability between individuals; elimination was dose-dependent and consistent with target-mediated drug disposition (TMDD) (
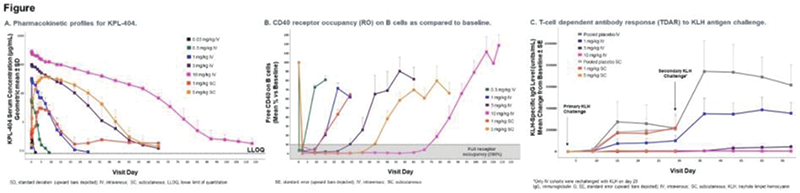
Conclusion: The safety and tolerability data and the PK/PD profile of KPL-404 support further investigation of KPL-404 in a broad range of autoimmune diseases, including rheumatoid arthritis. These data support the optionality for studying chronic KPL-404 dosing in patients with subcutaneous and/or intravenous administration.
Disclosure of Interests: Manoj Samant Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp., Alistair Wheeler Consultant of: Kiniksa Pharmaceuticals Corp., Guang-Liang Jiang Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp., Moses Njenga Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp., Madeline Spiers Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp., Arian Pano Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp., John F. Paolini Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp.