

Background: An unmet need remains in patients with failure and/or inadequate response (IR) to biological disease-modifying antirheumatic drugs (bDMARD-IR) and/or Janus kinase inhibitors (JAKi-IR). The CD40/CD40L (CD154) costimulatory pathway is linked to inflammation and joint destruction in RA via production of autoantibodies and inflammatory mediators. KPL-404 is a humanized IgG4 antibody engineered to bind CD40 without triggering Fc effector functions (Muralidharan, 2019), which are known to have been associated with thromboembolic events seen in the first generation of CD40L-targeting therapies.
In a first-in-human Phase 1 single ascending dose study, 52 healthy volunteers received single doses of KPL-404 administered either subcutaneously (SC) or intravenously (IV) with no dose-limiting safety findings, infectious episodes, or toxicities (Samant, 2021). The study demonstrated that with 10 mg/kg IV, full receptor occupancy (RO) was observed through day 71, and there was complete suppression of T-cell dependent antibody response (TDAR) to keyhole limpet hemocyanin challenge on day 1 and re-challenge on day 29 through day 57. With 5 mg/kg SC, full RO was observed through day 43, and there was complete suppression of TDAR through at least day 29. Complete suppression of ADA to KPL-404, an independent indicator of target engagement, was also observed while KPL-404 serum concentrations were above approximately 0.1 to 0.2 µg/mL and continued for at least 50 days and 57 days after 5 mg/kg SC and 10 mg/kg IV administration, respectively.
Objectives: Using Phase 1 and nonclinical data, identify chronic dosing regimens anticipated to yield PK in the sub-therapeutic, therapeutic, and supra-therapeutic ranges to be utilized in a Multiple Ascending Dose Phase 2 Study.
Methods: A PK model was used to simulate multiple dosing scenarios, including: 2.5, 5, and 10 mg/kg SC qwk, q2wk, and q4wk, as well as 10 mg/kg IV q4wk. The model was used to identify optimal Phase 2 dosing schedules by generating 1000 virtual subjects using the typical parameter estimates with between-subject variability included.
Results: Following SC administration, all subjects were predicted to achieve complete ADA suppression for the full dosing interval at/above 2.5 mg/kg SC q2wk. At 2 mg/kg SC q2wk (starting dose level), simulated steady-state 8-week data predicted PK in a sub-therapeutic range for most subjects and an approximately 31- and 18-fold safety margin relative to preclinical NOAEL dose. At 5 mg/kg SC q2wk, 100% of patients were predicted to be in a therapeutic range, indicating a potential practical efficacious dose level. At 10 mg/kg SC q2wk, 100% of patients were predicted to be in the supratherapeutic range.
These results support a Multiple Ascending Dose (MAD) Phase 2 study design, with PK lead-in comprised of 3 Cohorts at 2, 5, or 10 mg/kg SC q2wk (each randomized 6:2) and Proof-of-Concept phase (Cohort 4) comprised of 48-60 subjects randomized 1:1:1 to 10 mg/kg, 5 mg/kg, and placebo SC q2wk. The ongoing study will evaluate efficacy (Disease Activity of 28 joints using C-reactive protein [DAS28-CRP]), safety, PK, and pharmacodynamics (PD) of escalating doses levels of KPL-404 compared with placebo in patients with moderate to severe RA (bDMARD-IR or JAKi-IR). The study also allows the flexibility of optional cohorts including additional dosing regimens and/or subpopulations identified based on clinical response and biomarkers.
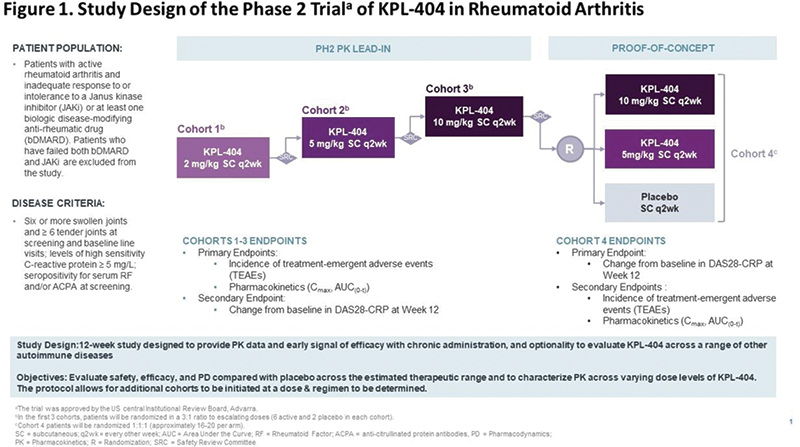
Conclusion: Inhibition of the CD40-CD154 co-stimulatory interaction holds promise for the management of a spectrum of autoimmune diseases. KPL-404 demonstrated prolonged absorption/excretion capable of suppressing TDAR for extended periods allowing for use of extended dosing intervals irrespective of IV or SC dosing. These analyses supported the design of the ongoing Phase 2 study assessing the efficacy and safety KPL-404 in RA.
REFERENCES:
[1]Muralidharan S et al. 2019. Poster at Keystone Symposia
[2]Samant M et al. Arthritis Rheumatol. 2021; 73(suppl 10)
Disclosure of Interests: Anastassia Papandrikopoulou Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp., Gerd Rüdiger Burmester Speakers bureau: Abbvie, Amgen, BMS, Lilly, MSD, Pfizer, Roche, Sanofi, Consultant of: Abbvie, Amgen, BMS, Kiniksa, Lilly, MSD, Pfizer, Roche, Sanofi, Fang Fang Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp., Alan Kivitz Shareholder of: Amgen, Gilead Sciences, Inc., GlaxoSmithKline, Novartis, Pfizer, Sanofi,, Speakers bureau: AbbVie, Celgene, Flexion, Genzyme, GlaxoSmithKline, Lilly, Merck, Novartis, Pfizer, Sanofi, UCB, Horizon, Consultant of: AbbVie, Boehringer Ingelheim, Flexion, Gilead Sciences, Inc., Janssen, Pfizer, Sanofi, SUN Pharma Advanced Research, Moses Njenga Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp., Arian Pano Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp., Costantino Pitzalis Speakers bureau: Abbott/AbbVie, Astra-
Zeneca/MedImmune, BMS, Janssen/J&J, MSD, Pfizer, Roche/Genentech/Chugai, UCB.,, Consultant of: Abbott/AbbVie, Astellas, Astra-Zeneca/MedImmune, BMS, CelGene, Grunenthal, GSK,
Janssen/J&J, Kiniksa, MSD, Pfizer, Sanofi, Roche / Genentech / Chugai, UCB., Grant/research support from: Abbott/AbbVie, Astellas, Astra-Zeneca/MedImmune, BMS, Janssen/J&J, MSD, Pfizer, Roche/Genentech/Chugai, UCB., Manoj Samant Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp., Steve Schmitz Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp., Madeline Spiers Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp., Eben Tessari Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp., John Ziemniak Consultant of: Kiniksa Pharmaceuticals, Ltd., John F. Paolini Shareholder of: Kiniksa Pharmaceuticals Corp., Employee of: Kiniksa Pharmaceuticals Corp.