

Background: Obesity is a risk factor for worse overall health in people with ankylosing spondylitis (AS) 1 . The German non-interventional study AQUILA provides real-world data in AS on the influence of body mass index (BMI) on therapeutic effectiveness and safety under treatment with secukinumab, a fully human monoclonal antibody that selectively inhibits IL-17A.
Objectives: The aims of this interim analysis are to describe selected baseline (BL) demographics and to evaluate secukinumab treatment outcomes on disease activity and global functioning and health and to report safety profile depending on the BMI of AS patients (pts).
Methods: AQUILA is an ongoing, multi-center, non-interventional study including up to 3000 pts with active AS or psoriatic arthritis. Pts were observed from BL up to week (w) 52 according to clinical routine. Real-world data were assessed prospectively and analyzed as observed. Validated questionnaires were used to collect data on disease activity (Bath Ankylosing Spondylitis Disease Activity Index, BASDAI) and global functioning and health (Assessment of SpondyloArthritis-Health Index, ASAS-HI). For calculation of proportion of pts that experienced (serious) adverse events ((S)AEs), all AS pts were included that received at least one dose of secukinumab. This interim analysis focuses on BMI subgroups ≤25 kg/m 2 (normal weight), > 25 to ≤30 kg/m 2 (overweight) and > 30 kg/m 2 (obese) in AS pts.
Results: At BL, BMI data were available for 667 AS pts: 33.6% (n=224) normal weight, 39.9% (n=266) overweight and 26.5% (n=177) obese AS pts (
Overview of baseline characteristics in AS pts depending on BMI
| Demographics | BMI ≤25 kg/m 2 (N=224) | BMI >25 to ≤30 kg/m 2 (N=266) | BMI >30 kg/m 2 (N=177) |
|---|---|---|---|
| Male* | 123 (54.9) | 178 (66.9) | 94 (53.1) |
| Age, years** | 43.3 (12.1) | 47.5 (12.3) | 49.2 (11.0) |
| BASDAI** | 4.8 (2.0) | 5.5 (1.8) | 5.5 (2.0) |
| ASAS-HI** | 7.4 (3.7) | 7.7 (3.3) | 8.1 (3.6) |
| Comorbidities/EAMs* | |||
| Heart-related disease | 4 (1.8) | 12 (4.5) | 12 (6.8) |
| Coronary heart disease | 4 (1.8) | 10 (3.8) | 8 (4.5) |
| Stroke | 1 (0.4) | 0 (0.0) | 2 (1.1) |
| Heart insufficiency | 1 (0.4) | 4 (1.5) | 8 (4.5) |
| Uveitis | 11 (4.9) | 17 (6.4) | 13 (7.3) |
| Depression | 88 (55.3) | 121 (58.2) | 73 (54.9) |
*variables are given as n (%); **variables given as mean (SD)
Mean BASDAI developed similarly over time with lowest scores for normal weight and highest scores for obese AS pts (
Disease activity and global functioning under secukinumab treatment in AS pts stratified by BMI
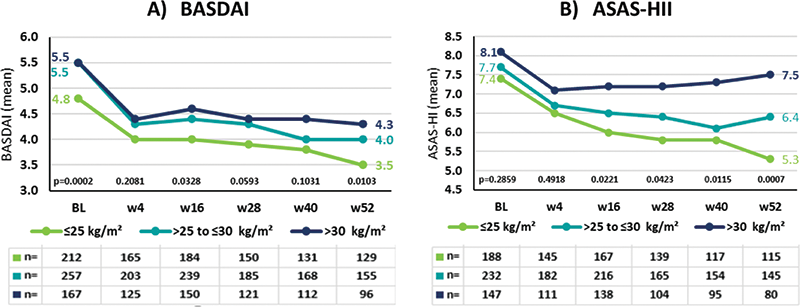
Mean ASAS-HI at BL was similar for all BMI subgroups (
≤25
: 7.4; >
25-≤30
: 7.7;
>30
: 8.1); the best improvement was observed in normal weight, the least in obese AS pts (
The occurrence of AEs/SAEs with or without suspected relationship to secukinumab increased with increasing BMI. For example, the percentage of SAEs in normal weight was 21%, in overweight 26.7% and in obese AS pts 30.9% (data not shown). There were no events with fatal outcome or unexpected safety signals in either subgroup.
Conclusion: In a real-world setting, secukinumab improved disease activity and global functioning and health in all BMI subgroups of AS pts; normal weight AS pts had numerically better ASAS-HI and BASDAI scores than obese AS pts. Altogether, real-world data of this interim analysis show that secukinumab is an effective treatment with a favorable safety profile up to 52 weeks in AS pts in all BMI subgroups.
REFERENCES:
[1]Chen, C.-H. , et al. International Journal of Rheumatic Diseases 23 , 1165-1174 (2020).
Disclosure of Interests: Uta Kiltz Consultant of: AbbVie, Amgen, Biogen, Chugai, Eli Lilly, Gilead, GSK, Grünenthal, Hexal, Janssen, MSD, Novartis, Pfizer, Roche, UCB, Grant/research support from: AbbVie, Amgen, Biogen, Chugai, Eli Lilly, Gilead, GSK, Grünenthal, Hexal, Janssen, MSD, Novartis, Pfizer, Roche, UCB, Jan Brandt-Juergens Consultant of: Abbvie, Affibody, BMS, Gilead, Janssen, Lilly, Medac, MSD, Novartis, Pfizer, Roche, Sanofi-Aventis, UCB, Peter Kästner Consultant of: Chugai, Novartis, Elke Riechers Consultant of: AbbVie, Chugai, Novartis, UCB, Grant/research support from: AbbVie, Chugai, Novartis, UCB, Pfizer, Daniel Peterlik Employee of: Novartis, Christina Budden Employee of: Novartis, Hans-Peter Tony Consultant of: AbbVie, Astra-Zeneca, BMS, Chugai, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi