

Background: Psoriatic arthritis (PsA), a chronic inflammatory disease characterized by peripheral arthritis, axial inflammation, dactylitis, enthesitis, and skin/nail psoriasis, is associated with reduced health-related quality of life (HRQoL).
Objectives: To assess long-term effect of guselkumab (GUS), a human monoclonal antibody that selectively targets the interleukin (IL)-23p19 subunit, on HRQoL of bio-naïve PsA patients (pts) who participated in the Phase 3 2-year DISCOVER-2 trial. 1
Methods: Pts with active PsA despite nonbiologic disease-modifying antirheumatic drugs (DMARDs) and/or nonsteroidal anti-inflammatory drugs (NSAIDs) received GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or placebo (PBO). At W24, PBO pts crossed over to GUS 100 mg Q4W. HRQoL was assessed using the pt-reported EuroQoL-5 Dimension-5 Level (EQ-5D-5L) questionnaire index and EuroQol Visual Analog Scale (EQ-VAS), widely used and complimentary tools that allow pts to provide a global assessment of their HRQoL. The EQ-5D-5L index assesses mobility, self-care, usual activities, pain/discomfort, and anxiety/depression; an index score is derived ranging from 0 (death) to 1 (perfect health). 2 EQ-VAS assesses pt health state on a scale of 0-100, with higher scores indicating better health. Using mixed effects models for repeated measures (MMRM), least squares (LS) mean changes from baseline in the EQ-5D-5L index and EQ-VAS through W100 were assessed. Observed changes from baseline were evaluated; in pts who met treatment failure rules before W24 and in pts who discontinued with missing data after W24, changes from baseline were imputed as 0.
Results: GUS-treated pts achieved greater improvements in pt-reported health status than PBO at both W16 and W24 when evaluated using both the EQ-5D-5L index score and the EQ-VAS. The improvements by GUS in EQ-5D-5L index scores through W24 (0.12 for GUS Q4W/Q8W vs 0.05 for PBO; each nominal p<0.0001) were maintained with continued GUS through 2 years (0.15 for GUS Q4W/Q8W) (
LS mean change from baseline through W100 in EQ-5D-5L index
| GUS 100mg Q4W(W0-100) | GUS 100mg Q8W(W0-100) | PBO → GUS 100 mg Q4W | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PBO(W0-24) | GUS(W24-100) | ||||||||
| Week | 16 | 24 | 100 | 16 | 24 | 100 | 16 | 24 | 100 |
| N | 243 | 244 | 243 | 247 | 246 | 248 | 244 | 244 | 244 |
| LS mean change (95% CI) | 0.10 (0.09,0.12) | 0.12 (0.1,0.13) | 0.15 (0.13,0.16) | 0.11 (0.1,0.13) | 0.12 (0.1,0.13) | 0.15 (0.13,0.17) | 0.06 (0.04,0.07) | 0.05 (0.04,0.07) | 0.14 (0.12,0.16) |
| Diff vs. PBO | 0.04 (0.02,0.06) | 0.06 (0.04,0.09) | -- | 0.05 (0.03,0.07) | 0.06 (0.04,0.08) | -- | -- | -- | -- |
| Nominal p-value | <0.0001 | <0.0001 | -- | <0.0001 | <0.0001 | -- | -- | -- | -- |
CI=Confidence interval; Diff=Difference
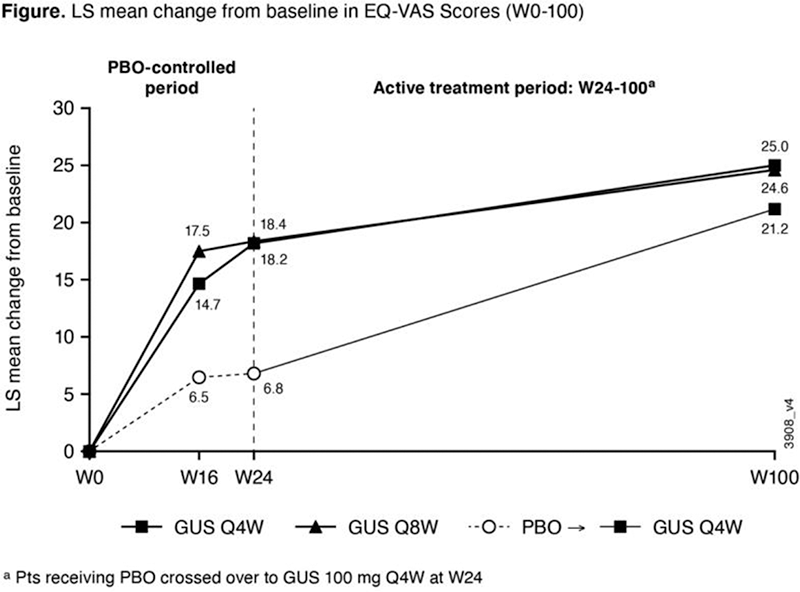
Conclusion: In bio-naïve pts with active PsA receiving GUS, earlier improvements (at the first timepoint assessed) in self-reported HRQoL measures were sustained through 2 years.
REFERENCES:
[1]Mease PJ, et al. Lancet. 2020;395:1126–36.
[2]EuroQol Group. 1990;16:199-208.
Disclosure of Interests: Jeffrey Curtis Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, CorEvitas, Eli Lilly, Janssen, Myriad, Novartis, Pfizer, Sanofi, and UCB, Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, CorEvitas, Eli Lilly, Janssen, Myriad, Novartis, Pfizer, Sanofi, and UCB, Iain McInnes Shareholder of: Causeway Therapeutics, and Evelo Compugen, Consultant of: Astra Zeneca, AbbVie, Amgen, Bristol-Myers Squibb, Cabaletta, Compugen, Eli Lilly, Gilead, GSK, Janssen, Novartis, Pfizer, Roche, Sanofi, and UCB, Grant/research support from: Astra Zeneca, Amgen, Bristol-Myers Squibb, Eli Lilly, GSK, Janssen, Novartis, Roche, and UCB, Proton Rahman Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer, and UCB, Grant/research support from: Janssen and Novartis, Dafna D Gladman Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB, Grant/research support from: AbbVie, Amgen, Eli Lilly, Janssen, Pfizer, and UCB, Feifei Yang Shareholder of: Johnson & Johnson, Employee of: Janssen Global Services, LLC (a wholly owned subsidiary of Johnson & Johnson), Steve Peterson Shareholder of: Johnson & Johnson, Employee of: Janssen Global Services, LLC (a wholly owned subsidiary of Johnson & Johnson), Alexa Kollmeier Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC (a wholly owned subsidiary of Johnson & Johnson), Natalie Shiff Shareholder of: AbbVie, Gilead, and Johnson & Johnson, Employee of: Janssen Scientific Affairs, LLC (a wholly owned subsidiary of Johnson & Johnson), Chenglong Han Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC (a wholly owned subsidiary of Johnson & Johnson), May Shawi Shareholder of: Johnson & Johnson, Employee of: Immunology Global Medical Affairs, Janssen Pharmaceutical Companies (a wholly owned subsidiary of Johnson & Johnson), William Tillett Speakers bureau: AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, and UCB, Consultant of: AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, and UCB, Grant/research support from: AbbVie, Amgen, Eli Lilly, Janssen, and UCB, Philip J Mease Speakers bureau: AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, SUN Pharma, and UCB, Consultant of: AbbVie, Aclaris, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, GSK, Inmagene, Janssen, Novartis, Pfizer, SUN Pharma, and UCB, Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, SUN Pharma, and UCB