

Background: Upadacitinib (UPA) is an inhibitor of JAK kinases recently approved by EMA for the treatment of psoriatic arthritis (PsA) in Europe (January 2021) 1. UPA has shown efficacy in refractory patients to anti-TNF 2.
Objectives: A ) to assess efficacy and safety of UPA in the first cases in Spain in clinical practice. B ) to compare the profile of clinical practice patients with clinical trial of UPA in PsA refractory to biologics 2 .
Methods: Study of 39 patients of clinical practice with PsA treated with UPA in Spain. The diagnosis of PsA was made using CASPAR criteria. Patients who received at least one dose of UPA were included. Results are expressed as percentage, mean±SD or median [IRQ].
Results:
39
patients (29♀/10♂), mean age of 51.5 ± 11.4 years (
| CLINICAL PRACTICE N=39 | CLINICAL TRIAL N=211 | p | |
|---|---|---|---|
| Baseline demographic parameters | |||
| Age, years (mean±SD) | 51.5±11.4 | 53.0 ± 12.0 | 0.4706 |
| Sex, n (%) female | 29 (74.4) | 113 (53.6) | 0.016 |
| Disease Characteristics | |||
| Duration of psoriatic arthritis, year (mean±SD) | 12.41±8.68 | 9.5 ± 8.4 | 0.0499 |
| HAQ-DI | 1.10± 0.42 | 1.10 ± 0.6 | 1.000 |
| Swollen joint count, mean±SD | 6±7.29 | 11.3 ± 8.2 | < 0.001 |
| Painful joint count, mean±SD | 7.48±7.58 | 24.9 ± 17.3 | < 0.001 |
| Enthesitis, n (%) | 23 (59.0) MASES | 172 (81.5) SPARCC | |
| Dactylitis, n (%) | 14 (35.9) | 55(26.1) | 0.217 |
| PASI score, mean±SD | 2.72±2.32 | 10.1 ± 9.2 | < 0.001 |
| CRP (mg/L) | 11.1±18.86 | 11.2 ± 18.5 | 1.000 |
| Oral glucocorticoid use, n (%) | 17 (43.6) | 22 (10.4) | < 0.001 |
| Concomitant synthetic DMARDs, n (%) | 16(41) | 98 (46,4) | 0.532 |
| Previous use of biological DMARDs, n (%) | 39(100) | 195 (92.4) | 0.075 |
| Number of prior failed biologic DMARDs, n(%) | |||
| 1 | 3(7.7) | 135 (63.7) | <0.001 |
| 2 | 4(10.3) | 35 (16.5) | 0.383 |
| ≥3 | 32(82) | 24 (11.3) | <0.001 |
| UPA in monotherapy, n (%) | 23(59) | 113 (53.6) | 0.531 |
HAQ-DI Health Assessment Questionnaire-Disability Index, PASI Psoriasis Area Severity Index, CRP C-reactive protein, DMARD disease-modifying antirheumatic drug
Prior to UPA, most patients (59%) had received oral prednisone or equivalent (max 9.03±12.12mg/d), synthetic immunosuppressants (mean1.8±0.9) and biological therapy (TB) (4.5±2.1). TB were as follows: etanercept (25), adalimumab (28), infliximab (12), golimumab (16), certolizumab (15), secukinumab (29), ustekinumab (21) Abatacept (2), brodalumab (1) and ixekizumab (17). Apremilast was used in 13, Tofacitinib in 11 and filgotinib in 1.
After a mean follow-up of 12.41± 8.68.3 years after the PsA diagnosis, UPA was started (15 mg/24 h), 43.6% associated prednisone (7.35±3.36 mg/d). In 16 (41%) UPA was started in combined therapy: methotrexate (9), salazopyrin (3) and leflunomide (4); in the remaining 23 (59%), monotherapy was prescribed. At UPA onset patients presented peripheral arthritis (76.9%), axial involvement (35.8%), skin involvement (25.6%), enthesitis (41%), and dactylitis (10.3%).
Patients of clinical practice compared with clinical trial there were more women, have a longer duration of PsA, and received a higher proportion of previous TB (
After a median follow-up of 4.28 ± 2.6 months, patients showed prompt improvement in activity indexes (DAS28, DAPSA) (
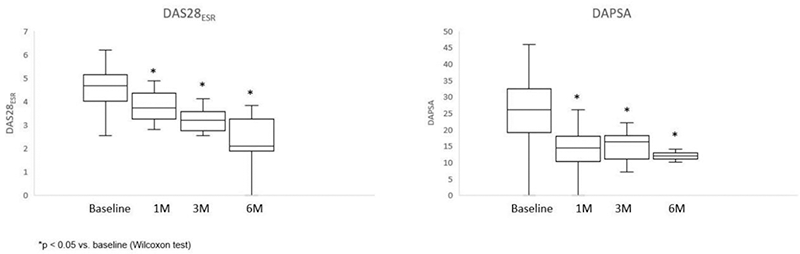
No serious events were reported. Minor side effects were reported in 7 patients (17.9%), and UPA was discontinued in 9 due to inefficiency.
Conclusion: In this preliminary study, first patients of clinical practice in Spain with UPA in PsA had a longer evolution and received a greater number of TB than those of clinical trial. As in the UPA clinical trial, it seems effective, rapid and relatively safe in daily clinical practice for refractory PsA.
REFERENCES:
[1]
[2]Mease PJ, et al. Ann Rheum Dis 2021; 80 :312–320
Disclosure of Interests: None declared