

Background: Chronic inflammation in psoriatic arthritis (PsA) may trigger both peripheral and central sensitization via central modifications of pain pathways that can lead to disconnection between tender and swollen joint count. This can result in increased difficulties for the clinician in the assessment of the disease and response to treatment.
Objectives: To study the impact of tender to swollen joint count ratio (TSR) on treatment response to a first course of biologic disease-modifying antirheumatic drug (bDMARD) therapy in PsA patients.
Methods: Observational study including PsA patients under bDMARD, followed with clinical and laboratory examination at baseline, 6 and 12 months of treatment. All patients meet the CASPAR classification criteria. TSR was defined as the tender joint count divided by the swollen joint count, using the 68/66 joint assessment. Patients with no tender nor swollen joints at baseline were excluded. TSR was categorized into 3 groups, based on the empirical distribution, with cuts corresponding to the 20 th and 70 th percentiles. Disease activity was assessed using the Clinical Disease Activity Index (CDAI), the Simplified Disease Activity Index (SDAI) and Disease Activity Score based on 28 joints (DAS28-CRP(4)). Individual time profiles were plotted within each TSR group. CDAI, SDAI and DAS28-CRP(4) individual time profiles within each TSR group were modelled by mixed-effects linear regression using the TSR group and time as fixed factors and a random factor at the intercept level (accounting for the intra-individual correlation structure). The identification of the statistically significant pairwise differences was obtained from the Tukey’s method for multiple comparisons.
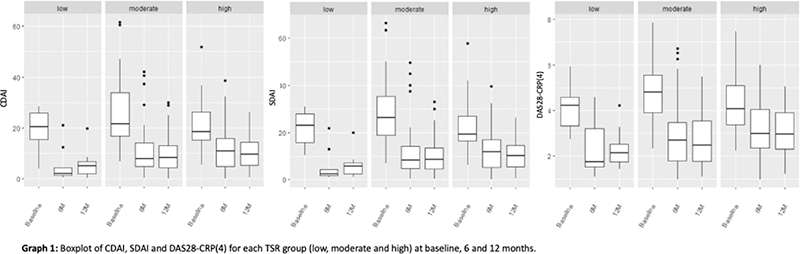
Results: We included 113 patients, 62 (54.0% females) with a mean age of 48.1±10.8 years-old at the start of the first bDMARD. Sixty-four patients (56.6%) had symmetric polyarthritis, 19 (16.8%) spondyloarthritis, 25 (22.1%) asymmetric oligoarthritis, 2 (1.8%) distal arthritis and 1 (0.9%) arthritis mutilans. Forty-three percent were under corticosteroid therapy and 57.5% under conventional synthetic DMARD (csDMARD) therapy at baseline (mostly methotrexate, in 45.1% of patients under csDMARD). Etanercept (n=35, 31.0%), adalimumab (n=34, 30.1%), golimumab (n=25, 22.1%), infliximab (n=6, 5.3%), certolizumab (5, 4.4%), secukinumab (n=8, 7.1%) were the bDMARD started in these patients. TSR was categorized into 3 groups, namely low [TSR < 1], moderate [1 ≤ TSR ≤ 2.2] and high [TSR > 2.2], with frequencies 15 (13.3%), 66 (58.4%) and 32 (28.3%), respectively. Whenever the number of tender joints was different from 0 and that of swollen joints equal to 0, patients were included in the group high TSR. All TSR groups, with initiation of bDMARD, showed significantly decreases at 6 months in CDAI (low: p=0.006, moderate: p<0.001, high: p<0.001), SDAI (low: p<0.001, moderate: p<0,001, high: p<0.001) and DAS28-CRP(4) (low: p<0.001, moderate: p<0.001, high: p<0.001). From 6 to 12 months of treatment, the differences were not significant in any of the groups (p>0.05). At baseline, CDAI, SDAI and DAS28-CRP(4) means did not differ between groups (p>0.05). There were also no differences in the means of outcome measures at 6 months as well as at 12 months of treatment (p>0.05). Despite this, patients with low baseline TSR had lower mean values of CDAI, SDAI and DAS28-CRP(4) at 6 and 12 months of treatment, consistent with a low disease activity.
Conclusion: To our knowledge this is the first study exploring the TSR on treatment response in samples of patients exclusively with PsA. All patients benefited from bDMARD therapy, regardless of the group, suggesting that TSR might not be a good predictor of treatment response in patients with PsA.
Disclosure of Interests: None declared