

Background: Gout affects more than 9 million Americans, 1 with some suffering so severely that everyday life becomes painful and difficult. 2 Pegloticase is an infused uricase-based medicine that significantly reduces serum uric acid (sUA) levels in patients suffering from treatment-resistant gout, 3 but the direct effect of treatment on quality of life (QoL) by response status has not yet been reported. 4
Objectives: To determine if the effect of pegloticase treatment on QoL and other patient-reported outcomes is significant compared to placebo, regardless of treatment response status.
Methods: This analysis utilized results from two parallel 6-month randomized phase 3 registrational clinical trials in which subjects were treated with 8 mg of pegloticase or placebo biweekly. Responders were those who maintained sUA levels <6 mg/dL for ≥80% of the time during Months 3 and 6 combined. Multiple QoL measurements were taken throughout the 6-month treatment period, including the Health Assessment Questionnaire (HAQ; Pain, Disability Index [DI], and Health), the 36-Item Short Form Health Survey Questionnaire (SF-36), and the number of swollen and/or tender joints. This analysis assessed the differences between three groups: treatment responders, treatment non-responders, and placebo-treated patients. The change from baseline to Week 25 in each assessment was analyzed using a mixed model for repeated measures adjusting for age, visit, presence of tophi at baseline, and the interaction between visit and group.
Results: A total of 36, 49, and 43 patients were included in the treatment responder, treatment non-responder, and placebo-treated groups, respectively. Significant differences in the change from baseline to Week 25 in HAQ-Pain, HAQ-DI, HAQ-Health, and SF-36 Physical Component Score (PCS) between treatment responders and placebo-treated patients were observed (all P ≤ 0.006,
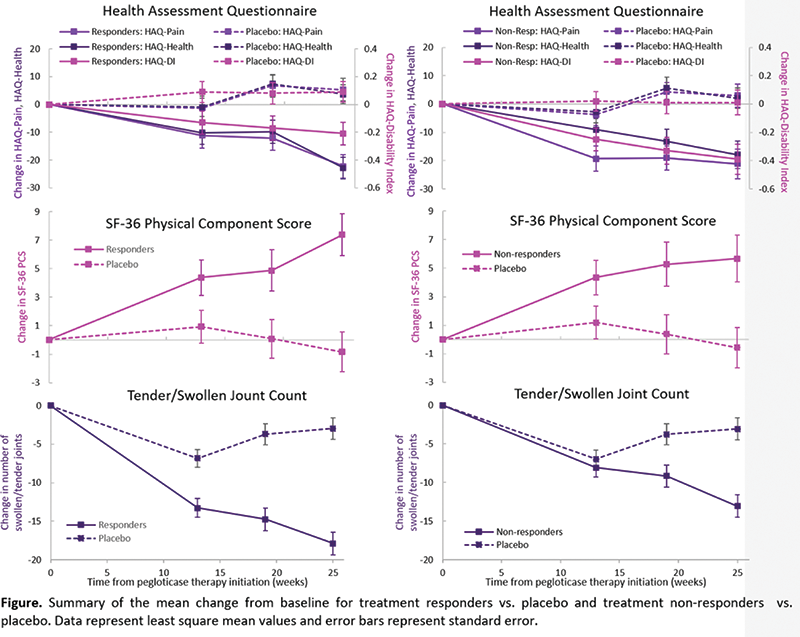
Conclusion: In the Phase 3 registration trials of pegloticase used as monotherapy for uncontrolled gout, QoL parameters improved among pegloticase-treated patients regardless of response status.
REFERENCES:
[1]Chen-Xu M, et al. Arthritis Rheumatol 2019;71:991-9.
[2]Becker MA, et al. J Rheumatol 2009;36:1041-8.
[3]Sundy JS, et al. JAMA 2011;306:711-20.
[4]Strand V, et al. J Rheumatol 2012;39:1450-7.
Disclosure of Interests: Jacy Sparks: None declared, Katie Obermeyer Shareholder of: Horizon Therapeutics., Employee of: Horizon Therapeutics., Brian LaMoreaux Shareholder of: Horizon Therapeutics., Employee of: Horizon Therapeutics.