

Background: By definition, uncontrolled gout (UG) cannot be managed with oral urate lowering therapies (ULTs) and is associated with substantial morbidity. UG, also known as refractory gout, results in escalated treatment and management. Recent American College of Rheumatology guidelines recommend treating gout to serum uric acid targets; if targets are not achieved or patients continue to have symptoms, pegloticase is recommended. There is a paucity of data documenting the clinical and economic burden of UG patients.
Objectives: Assess clinical outcomes and healthcare resource utilization (HCRU) of UG prior to pegloticase initiation.
Methods: A retrospective observational database analysis was conducted among patients initiating pegloticase between April 1, 2011 and August 31, 2020 using the PharMetrics Plus database. Eligible subjects had ≥1 pegloticase claim (first claim = index date) and continuous enrollment for 24 months prior to index. Relevant clinical and economic (HCRU) outcomes were evaluated over a 24-month pre-index period and compared between two different time intervals prior to index: time interval 1 (Day -720 to Day -361) and time interval 2 (Day -360 to Day -1). Assessment of comorbid disease burden included Charlson Comorbidity Index (CCI) and relevant health conditions. Dependent pairwise comparisons were conducted to compare clinical and economic outcomes between time intervals prior to pegloticase initiation. To assess statistical differences, paired t-tests (continuous variables) or McNemar’s tests (categorical variables) were used.
Results: Of the 408 eligible subjects, most were male (88.5%), with an average age (SD) of 55.2 (11.3) years, 66.9% were between the ages of 45-64 years and 78.2% had a preferred provider organization (PPO) health plan. Most often (34.8% of patients), a rheumatologist was associated with initiation of pegloticase therapy, while primary care physicians accounted for 23.8% of initiations. Mean (SD) CCI score was 2.4 (2.4) with 37.3% of subjects having a CCI score of >3. Prevalence of relevant health conditions over the 24-month pre-index period included tophi (62.5%), urolithiasis (8.6%), chronic kidney disease (34.6%) and chronic pain/fibromyalgia (76.5%), all of which significantly increased from time interval 1 (Day -720 to Day -361) to time interval 2 (Day -360 to Day -1) prior to pegloticase initiation (
Relevant Health Conditions and Disease-specific Health Care Resource Utilization (HCRU)
| Overall N= 408 | Time Interval 1 | Time Interval 2 | |
|---|---|---|---|
| Tophi | 62.5% | 15.4% | 61.5%*** |
| Urolithiasis | 8.6% | 4.2% | 6.9%* |
| Chronic kidney disease | 34.6% | 22.5% | 31.6%*** |
| Cardiovascular disease | 32.6% | 21.3% | 28.4%** |
| Type 2 diabetes mellitus | 31.4% | 23.3% | 28.9%** |
| Hypertension | 76.2% | 58.1% | 70.3%*** |
| ≥1 gout flare | 87.7% | 48.5% | 83.8%*** |
| Mean number of gout flare (SD) | 3.5 (2.4) | 1.0 | 2.1*** |
| Gout-related medications ≥1 claim for colchicine | 56% | 39.5% | 63.7%*** |
| ≥1 claim for opioids | 71% | 52.9% | 60.3%* |
| ≥1 claim for oral corticosteroids | 80% | 50.2% | 75.7%*** |
| ≥1 claim for injectable corticosteroids | 64% | 38.5% | 53.7%*** |
†Time Interval 1: Day -720 to Day -361 prior to pegloticase initiation; ††Time Interval 2: Day -360 to Day -1 prior to pegloticase initiation
***, p<0.0001; **, p<0.001; *, p<0.05
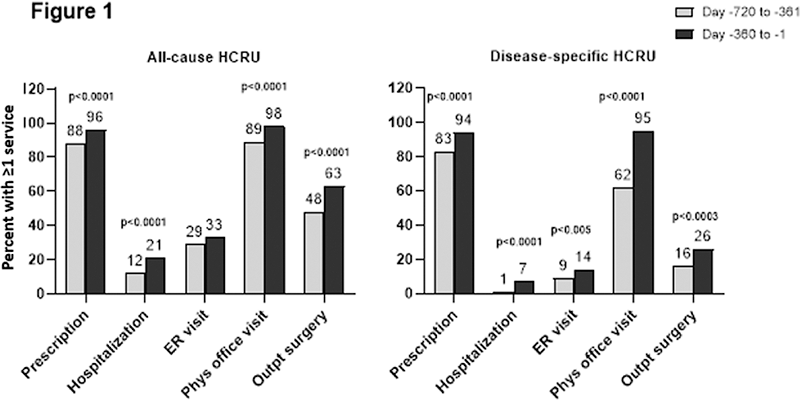
Conclusion: Overall, these data demonstrate the progressive nature of UG as confirmed by significant increases in gout-related conditions and healthcare resource utilization prior to pegloticase initiation. Further research is needed on healthcare resource utilization among patients with UG post-pegloticase use.
Disclosure of Interests: Robert Morlock Consultant of: Horizon Therapeutics, Victoria Divino Grant/research support from: Horizon Therapeutics, Mitchell DeKoven Grant/research support from: Horizon Therapeutics, Brian LaMoreaux Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Atsuko Powers Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Naina Barretto Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Robert Holt Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Stephanie Taylor Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics