

Background: Recombinant uricase such as pegloticase are indicated for chronic gouty arthritis who have failed to achieve serum urate level <300μmol/L even though receiving conventional urate-lowering drugs. Rasburicase which is currently approved at a dosage of 0.2 mg/(kg.d) for 5 days for the prevention of tumor lysis syndrome in pediatric patients with hematological tumors, is the only available uricase in China at present.
Objectives: To evaluate the efficacy and safety of low-dose rasburicase in refractory chronic gouty arthritis.
Methods: We retrospectively collected data of 17 patients with refractory chronic gouty arthritis who were treated with rasburicase from January 2021 to September 2021 at Sun Yat-sen Memorial Hospital, Sun Yat-sen University. The refractory chronic gouty arthritis was defined as serum urate level was still more than 300μmol/L and dual-energy CT showed the volume of urate more than 10 cm 3 , even though the allopurinol, febuxostat, and/or benzbromarone of the maximum tolerable amount were received for more than 3 months. Rasburicase was added every four weeks (week 0, week 4, and week 8) with a dosage of 1.5 mg/d for three consecutive days, on the basis of daily oral urate-lowering drugs of the maximum tolerable amount. The serum urate level was monitored. The primary outcome was the change in urate volume at week 12 compared to week 0 by dual-energy CT.
Results: ① Seventeen patients were recruited with 16 males (94%) and mean age 47±15 years old. The median gout course was 11 (6.5, 15) years with gout flares number 20 (11, 36) times in the previous year. At week 0 before the rasburicase add-on treatment, the mean serum urate was 652±94μmol/L and the median urate volume was 44 (21, 215) cm
3
(Table 1). ② Urate level after the rasburicase add-on treatment was significantly decreased than that before the treatment either at week 0, week 4, or week 8 (
The serum urate and urate volume before and after rasburicase add-on treatment. A: Paired comparison of serum urate level before and after each time of rasburicase administration; B. Reduction value and percentage of urate volume at week 12 for each patient. C&D: Dual-energy CT (DECT) showed a dramatical reduction in urate volume from week 0 (C, urate volume: 72.15 cm3) to week 12 (D, urate volume: 7.66 cm3). ***: p< 0.001; #: Received rasburicase at week 0 only; ##&###: Received rasburicase 7.5mg at week 0.
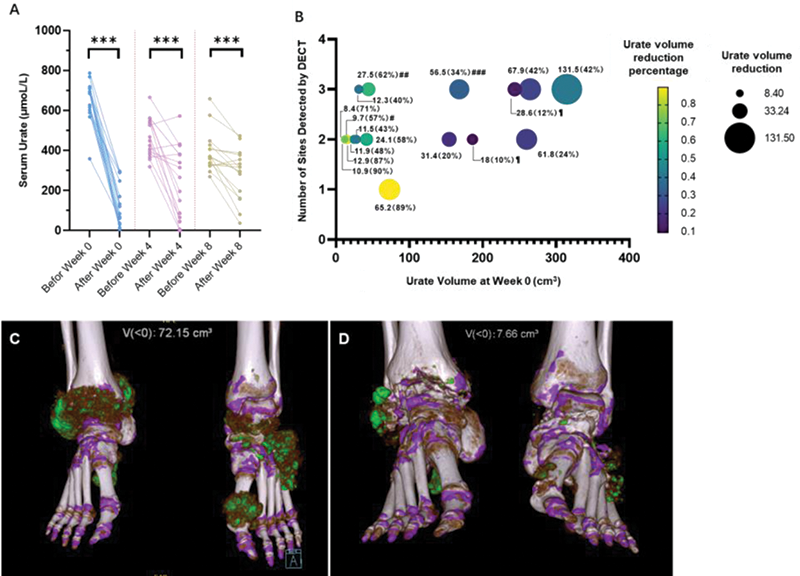
Conclusion: This pilot study shows rasburicase is well tolerated in patients with refractory chronic gouty arthritis and may be a reasonable option to effectively lower the urate burden of these patients, although this is an off-label use. Further prospective randomized controlled studies to verify the efficacy and safety are needed.
Funding: This study was funded by Yat-sen Clinical Research Project.
Disclosure of Interests: None declared