

Background: Positron emission tomography (PET) is one of the tools available for the diagnosis of extracranial large-vessel vasculitis (1-5 ). Tocilizumab (TCZ) has shown efficacy in large-vessel vasculitis (LVV) including GCA. However, the improvement objectified by imaging techniques after TCZ therapy in extracranial GCA patients is controversial.
Objectives: To assess the effectiveness of TCZ improving the wall vessel inflammation by PET in GCA patients with large-vessel involvement.
Methods: Observational, multicenter study of 101 GCA patients treated with TCZ. GCA was diagnosed according to: a ) ACR criteria, and/or b ) biopsy of temporal artery, and/or c ) presence of signs of vessel wall inflammation by PET, defined by the presence of vascular wall uptake of Fluorodeoxyglucose (FDG). Patients were divided into two subgroups: a ) with, and b ) without signs of improvement (partial or total) in the follow-up PET.
Results: We studied 101 patients (74 women/27 men; mean age 69.7±9.3 years). Main clinical features of GCA with and without PET improvement are shown in
Main features of 101 GCA patients treated with tocilizumab and with presence of signs of vessel wall inflammation by PET.
| With PET improvement (n=88 ) | Without PET improvement (n=13 ) | p | |
|---|---|---|---|
| Baseline characteristics at TCZ onset | |||
| General characteristics | |||
| Age(years), mean±SD | 70.6±9.1 | 63.8±9.2 | 0.014 |
| Sex, female/male (% female) | 67/21(76) | 7/6 (54) | 0.103 |
| Time from GCA diagnosis to TCZ onset (months), median [IQR] | 11 [4-24.2] | 4 [2-6] | 0.102 |
| Systemic manifestations, n (% ) | |||
| Fever, n (%) | 5 (6) | 2 (15) | 0.225 |
| Constitutional syndrome, n (%) | 36 (41) | 4 (31) | 0.466 |
| PmR, n (%) | 53 (60) | 9 (10) | 0.761 |
| Ischaemic manifestations, n (% ) | |||
| Visual involvement, n (%) | 2 (2) | 1 (1) | 0.342 |
| Headache, n (%) | 30 (34) | 3 (23) | 0.538 |
| Jaw claudication, n (%) | 8 (9) | 0 (0) | 0.592 |
| Laboratory data | |||
| ESR, mm 1st hour, median [IQR] | 38.0 ± 26.2 | 13.54 ± 9.9 | 0.001 |
| CRP, mg/dL, median [IQR] | 1.5 [0.7-2.4] | 1 [0.5-1.7] | 0.179 |
| Prednisone dose, mg/day, median [IQR] | 40.3 ± 19.4 | 21.9 ± 12.7 | 0.001 |
| Time from TCZ onset and follow-up PET (months) | 13.1±8.0 | 10.1±5.3 | 0.446 |
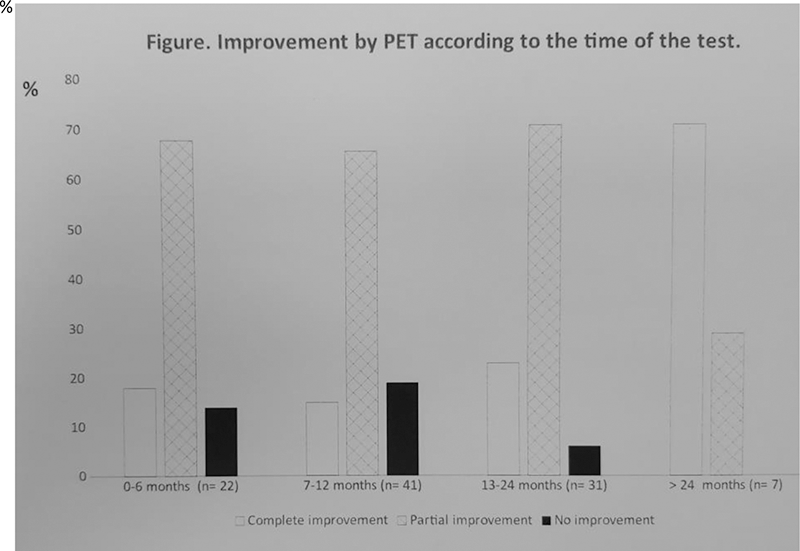
Conclusion: TCZ seems to be effective controlling GCA including vascular involvement detected by PET. However, the improvement observed by PET is most often partial, and rarely complete.
Improvement by PET according to the time of the test.
REFERENCES:
[1]Loricera J, et al. Rev Esp Med Nucl Imagen Mol. 2015; 34: 372-7. PMID: 26272121
[2]Loricera J, et al. Clin Exp Rheumatol. 2015; 33: S19-31. PMID: 25437450
[3]Prieto-Peña D, et al. Ther Adv Musculoskelet Dis. 2021; 13: 1759720X211020917. PMID: 34211589
[4]Martínez-Rodríguez I, et al. Semin Arthritis Rheum. 2018; 47: 530-537. PMID: 28967430
[5]Prieto-Peña D, et al. Semin Arthritis Rheum. 2019; 48: 720-727. PMID: 29903537
Acknowledgements: Tocilizumab in Giant Cell Arteritis Spanish Collaborative Group: Juan C. González Nieto (H. Gregorio Marañón), Juan R. de Dios (H.U. Araba), Esther Fernández (H. Clínico Universitario Virgen de la Arrixaca), Isabel de la Morena (H. Clínico Universitario de Valencia), Patricia Moya (H. Sant Pau), Roser Solans i Laqué (H. Valle de Hebrón), Eva Pérez Pampín (H.U. de Santiago), José L. Andréu (H.U. Puerta de Hierro), Marcelino Revenga (H. Ramón y Cajal), Juan P. Baldivieso Achá (H. U. de La Princesa), Eztizen Labrador (H. San Pedro), Andrea García-Valle (Complejo Asistencial Universitario de Palencia), Adela Gallego (Complejo Hospitalario Universitario de Badajoz), Carlota Iñíguez (H.U. Lucus Augusti), Cristina Hidalgo (Complejo Asistencial Universitario de Salamanca), Noemí Garrido-Puñal (H. Virgen del Rocío), Ruth López-González (Complejo Hospitalario de Zamora), José A. Román-Ivorra (H.U. y Politécnico La Fe), Sara Manrique (H. Regional de Málaga), Paz Collado (H.U. Severo Ochoa), Enrique Raya (H. San Cecilio), Valvanera Pinillos (H. San Pedro), Francisco Navarro (H. General Universitario de Elche), Alejandro Olivé-Marqués (H. Trías i Pujol), Francisco J. Toyos (H.U. Virgen Macarena), María L. Marena Rojas (H. La Mancha Centro), Antoni Juan Más (H.U. Son Llàtzer), Beatriz Arca (H.U. San Agustín), Carmen Ordás-Calvo (H. Cabueñes), María D. Boquet (H. Arnau de Vilanova), Noelia Álvarez-Rivas (H.U. Lucus Augusti), María L. Velloso-Feijoo (H.U. de Valme), Cristina Campos (H. General Universitario de Valencia), Íñigo Rúa-Figueroa (H. Doctor Negrín), Antonio García (H. Virgen de las Nieves), Carlos Vázquez (H. Miguel Servet), Pau Lluch (H. Mateu Orfila), Carmen Torres (Complejo Asistencial de Ávila), Cristina Luna (H.U. Nuestra Señora de la Candelaria), Elena Becerra (H.U. de Torrevieja), Nagore Fernández-Llanio (H. Arnáu de Vilanova), Arantxa Conesa (H.U. de Castellón), Eva Salgado (Complejo Hospitalario Universitario de Ourense).
Disclosure of Interests: Julio Sanchez-Martin: None declared, Javier Loricera: None declared, Santos Castañeda: None declared, Clara Moriano: None declared, J. Narváez: None declared, Vicente Aldasoro: None declared, Olga Maiz: None declared, Rafael Melero: None declared, Ignacio Villa-Blanco: None declared, Paloma Vela-Casasempere: None declared, Susana Romero-Yuste: None declared, Jose Luis Callejas-Rubio: None declared, Eugenio de Miguel: None declared, E. Galíndez-Agirregoikoa: None declared, Francisca Sivera: None declared, Carlos Fernández-López: None declared, Carles Galisteo: None declared, Iván Ferraz-Amaro: None declared, Lara Sanchez-Bilbao: None declared, Monica Calderón-Goercke: None declared, Jose Luis Hernández Hernández: None declared, Miguel A González-Gay Speakers bureau: Abbvie, Pfizer, Roche, Sanofi, Lilly, Celgene and MSD, Grant/research support from: Abbvie, MSD, Jansen and Roche, Ricardo Blanco Speakers bureau: Abbvie, Lilly, Pfizer, Roche, Bristol-Myers, Janssen, UCB Pharma and MSD, Grant/research support from: Abbvie, MSD and Roche