

Background: The availability of biosimilars has created a financial incentive to encourage non-medical switching if cheaper products are on the market. In patients with chronic inflammatory rheumatic diseases (CIRD), we have previously reported a relatively high retention rate after switching from originator etanercept to its biosimilar. However, this has been different in other studies and the reasons for non-adherence are poorly understood. Comorbidity has recently gained much attention in patients with CIRD and might be a reason for non-adherence.
Objectives: The aim of this study was to analyse the effectiveness and safety of systematic non-medical switching from originator adalimumab (ADA) to ADA ABP501 biosimilar (ABP) over 6 months in patients with CIRD and to investigate the influence of comorbidities on retention rates.
Methods: Patients with CIRD on originator ADA who switched to ABP subsequently from October 2018 onwards were identified from a large routine database and then followed for 6 months. The presence of comorbidities and disease characteristics as well as measures of disease activity, physical function and changes in treatment were documented at baseline (the time of switching from originator ADA to ABP), and at months 3 and 6. Longitudinal data including information on the clinical efficacy and safety of ABP, and the reasons for discontinuation were documented.
Results: A total of 111 CIRD patients on treatment with originator ADA were switched to the biosimilar ABP (
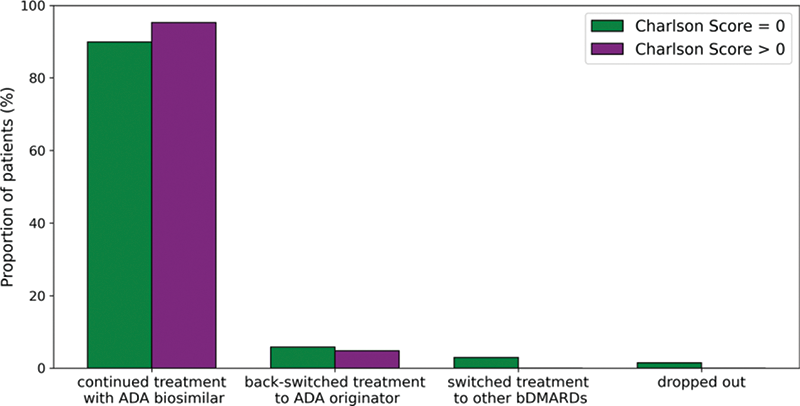
Treatment retention after 6 months stratified by the Charlson comorbidity score
Patients and disease characteristics
| RA | axSpA | PsA | Other | |
|---|---|---|---|---|
| N=23 | N=68 | N=15 | N=5 | |
| Age (years), mean (SD) | 65.1 (12.0) | 47.3 (13.1) | 51.1 (11.2) | 41.8 (14.2) |
| Women | 60.9% (14) | 32.4% (22) | 53.3% (8) | 40.0% (2) |
| Disease duration (years), median (IQR) | 4.0 (3.0-8.0) | 5.0 (2.0-8.0) | 4.0 (2.0-13.0) | 7.0 (4.0-7.0) |
| Duration originator ADA therapy (month), mean (SD) | 43.8 (28.6) | 39.4 (26.9) | 34.7 (29.0) | 60.9 (27.7) |
| Charlson score, mean (SD) | 1.8 (2.1) | 0.6 (1.1) | 0.7 (1.2) | 0.2 (0.4) |
| Gastroenterological comorbidities | 26.1% (6) | 22.1% (15) | 6.7% (1) | 0 |
| Hepatic comorbidities | 17.4% (4) | 2.9% (2) | 13.3% (2) | 0 |
| Hematological conditions | 8.7% (2) | 2.9% (2) | 13.3% (2) | 0 |
| Cardiovascular comorbidities | 60.9% (14) | 32.4% (22) | 33.3% (5) | 60.0% (3) |
| Neurological and psychological comorbidities | 8.7% (2) | 17.6% (12) | 33.3% (5) | 0 |
| Metabolic comorbidities | 21.7% (5) | 7.4% (5) | 26.7% (4) | 40.0% (2) |
| Osteoporosis | 43.5% (10) | 11.9% (8) | 6.7% (1) | 20.0% (1) |
| Lung diseases | 21.7% (5) | 8.8% (6) | 0 | 40.0% (2) |
| Skin diseases | 26.1% (6) | 26.5% (18) | 80.0% (12) | 20.0% (1) |
| Eye diseases | 8.7% (2) | 23.5% (16) | 6.7% (1) | 60.0% (3) |
| Kidney diseases | 13.0% (3) | 10.3% (7) | 0 | 40.0% (2) |
Conclusion: Comorbidity had no influence on the biosimilar retention rate after 6 months in this study but the majority of patients did not have Charlson scores > 0. However, disease activity and physical function tended to be worse among CIRD patients with comorbidity. Cardiovascular disease and osteoporosis were more often present in RA patients than in axSpA or PsA patients, while neurological and psychological comorbidities were more often observed in the latter.
Disclosure of Interests: Imke Redeker: None declared, Stefan Moustakis: None declared, Styliani Tsiami: None declared, Xenofon Baraliakos Speakers bureau: Abbvie, Pfizer, MSD, UCB, Novartis, Lilly, Galapagos, Hexal, Paid instructor for: Abbvie, Pfizer, MSD, UCB, Novartis, Lilly, Galapagos, Hexal, Consultant of: Abbvie, Pfizer, MSD, UCB, Novartis, Lilly, Galapagos, Hexal, Grant/research support from: Abbvie, Pfizer, MSD, UCB, Novartis, Lilly, Galapagos, Hexal, Ioana Andreica Speakers bureau: UCB, MSD, Novartis, Abbvie, Lilly, Janssen, SOBI, Consultant of: Lilly, Novartis, Galapagos, Amgen, Takkeda, SOBI, Grant/research support from: Lilly, Bjoern Buehring Speakers bureau: UCB, Amgen, Gilad/Galapagos, Biogen, Sanofi/Genzyme, Consultant of: UCB, Theramex, Gilead/Galapagos, Amgen, Abbvie, Juergen Braun Speakers bureau: Abbvie, Amgen, Biogen, BMS, Boehringer, Celltrion, Chugai, Fresenius, Hexal, Janssen, Lilly, Medac, MSD, Mylan, Mundipharma, Novartis, Pfizer und UCB, Consultant of: Abbvie, Amgen, Biogen, BMS, Boehringer, Celltrion, Chugai, Fresenius, Hexal, Janssen, Lilly, Medac, MSD, Mylan, Mundipharma, Novartis, Pfizer und UCB, Grant/research support from: Abbvie, Amgen, Biogen, BMS, Boehringer, Celltrion, Chugai, Fresenius, Hexal, Janssen, Lilly, Medac, MSD, Mylan, Mundipharma, Novartis, Pfizer und UCB, Uta Kiltz Speakers bureau: AbbVie, Biocad, Biogen, Chugai, Eli Lilly, Hexal, Janssen, MSD, Novartis, Pfizer, Roche, UCB, Consultant of: AbbVie, Biocad, Biogen, Chugai, Eli Lilly, Fresenius, Hexal, Janssen, MSD, Novartis, Pfizer, Roche, UCB, Grant/research support from: AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer.