

Background: The list of biological DMARDs (bDMARDS) and targeted synthetic DMARDs (tsDMARDs) approved to treat synovitis and extra-articular manifestations is long and constantly expanding. The choice of drug for the individual patient depends on multiple factors. 1) Among these, contraindications and warnings are pivotal to consider. 2), 3) However, a systematic collection of drug class specific warnings for the b/tsDMARDs based on regulatory drug approvals is lacking.
Objectives: Our aim was to make an overview of the different contraindications, boxed warnings and special warnings listed in the European Medicines Agency (EMA) summaries of product characteristics (SmPCs) and the U.S. Food and Drug Administration (FDA) Prescribing Information (PI) for b/tsDMARDs.
Methods: We included all b/tsDMARDs with an FDA and/or EMA approval for any of the diagnoses: rheumatoid arthritis (RA), psoriatic arthritis (PsA), spondylarthritis (SpA), juvenile idiopathic arthritis (JIA), uveitis, plaque psoriasis, inflammatory bowel diseases (IBD). The included drugs were tumor necrosis factor (TNF) inhibitors (inh), anti-CD20 (rituximab), inhibitors of interleukin (IL)-6 receptor (6R), IL-1, IL-17, IL-12/23, IL-23, CD80/86 (abatacept), janus kinase (JAK), the phosphodiesterase 4 (PDE4) (apremilast), and the α 4β7 antibody (vedolizumab)
We used the most recent SmPCs and PIs for the reference products in case of existing biosimilar products and extracted the information listed in the categories; contraindications, boxed warnings, and special warnings.
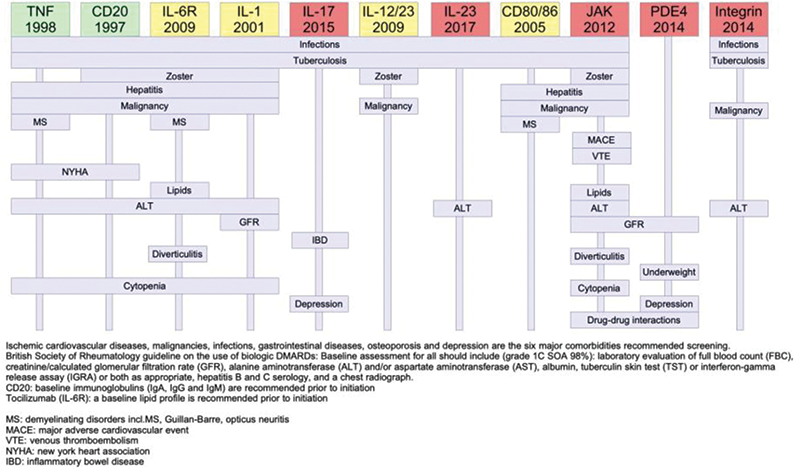
Results: There was a big difference between the year of first approval of the drugs spanning from 1998 to 2017. All drug classes except apremilast had warnings about infection. A warning about herpes zoster was listed specifically for rituximab, inhibitors of IL-6R, IL-1, IL-12/23 and JAK, while warning about hepatitis was listed for TNF inhibitors, rituximab, IL-6R inh., IL-1 inh., abatacept and JAK inh. All drug classes except IL-17 inh., IL-12/23 inh. and apremilast had warnings about malignancy. A warning about demyelinating disease was listed for TNF inh., IL-6R inh., and abatacept. Heart failure was specific for TNF inh. and rituximab while risk of major adverse cardiac events and venous thromboembolism was listed only for JAK inh. A recommendation regarding monitoring lipids was listed for inhibitors of IL-6R and JAK, monitoring liver enzymes was listed for all drug classes except IL-17 inh., IL-12/23 inh., abatacept and apremilast. Development of IBD was listed for IL-17 inh. and risk of diverticulitis was listed for inhibitor of IL-6R and JAK. Cytopenia was listed for rituximab, inhibitors of TNF, IL-6R, IL-1 and JAK. Depression was listed for IL-17 inh. and apremilast.
Conclusion: We made a systematic description of contraindications, boxed warnings and special warnings for b/tsDMARDs with a focus on drugs approved for use in chronic inflammatory arthritis and related disease entities. There were some associations between time of first drug approval and number of listed concerns. This, we presume, is because some of the rare events will not be found before several years after marketing. Group effects and specific concerns for individual drugs were identified.
Based on the warnings, we made a figure with clinically relevant conditions to consider when choosing a drug.
This systematic data collection could prove to be a useful decision tool for clinical practice to ensure patient safety and improve personalized drug choices.
REFERENCES:
[1]Smolen JS, et al. Ann Rheum Dis 2020;79:685–699. doi:10.1136/annrheumdis-2019-216655
[2]Sepriano A, et al. Ann Rheum Dis 2020;79:760–770. doi:10.1136/annrheumdis-2019-216653
[3]Holroyd CR, et al. Rheumatology, Volume 58, Issue 2, February 2019, Pages e3–e42,
Disclosure of Interests: Lykke Skaarup: None declared, Tue Wenzel Kragstrup Shareholder of: Co-founder and clinical developer in iBio tech ApS., Speakers bureau: Speaking fees from Pfizer, Bristol-Myers Squibb, Eli Lilly, Novartis, UCB, and Abbvie., Consultant of: Consultancy fees from Bristol-Myers Squibb and Gilead., Grant/research support from: Research grants from Gilead.