

Background: Decrease treatment persistence in rheumatoid arthritis (RA) patients has been associated with several factors, including number of previous biological DMARDs (bDMARDs), female gender and higher disease activity or lower function at baseline [1].
Objectives: Determine if drug discontinuation of bDMARDs differs by disease activity level at baseline in patients with RA in the Mexican Adverse Events Registry (BIOBADAMEX).
Methods: BIOBADAMEX is a Mexican ongoing cohort of patients using bDMARDs. In this analysis we included all patients with RA registered from 2016 to 2021 with at least two assessments. Survival on bDMARDs was estimated using Kaplan-Meier analysis. Predictors of discontinuation, including high disease activity defined as DAS28-ESR>5.1, were investigated by Cox regression analyses.
Results: Among 528 RA patients in the registry, 302 had at least two assessments. Of patients analyzed, 276 (91%) were women. At baseline, patients had a median (IQR) age of 52.7 (44-60) years old, median disease duration of 9.3 (4-16) months. A total of 142 (47%) had comorbidities, 34 (11%) had more than 2 morbidities. At baseline DAS28-ESR was 4.8 (4-6), 59 (20%) patients had low (DAS28-ESR<=3.2) and 130 (43%) had high disease activity. The most common bDMARDs received at baseline were abatacept 68 (23%), tocilizumab 59 (20%), adalimumab 50 (17%) and certolizumab 41 (14%). At the time of analysis, the median bDMARDs treatment duration was 17.2 (12-27) months. Overall, 130 (43%) patients had discontinued treatment, the most common causes of discontinuation were inefficacy in 64 patients, 15 for remission, 12 for adverse events and 26 for others.
Discontinuation rate curves in RA patients with high disease activity (DAS28 >5.1) and DAS28<=5.1
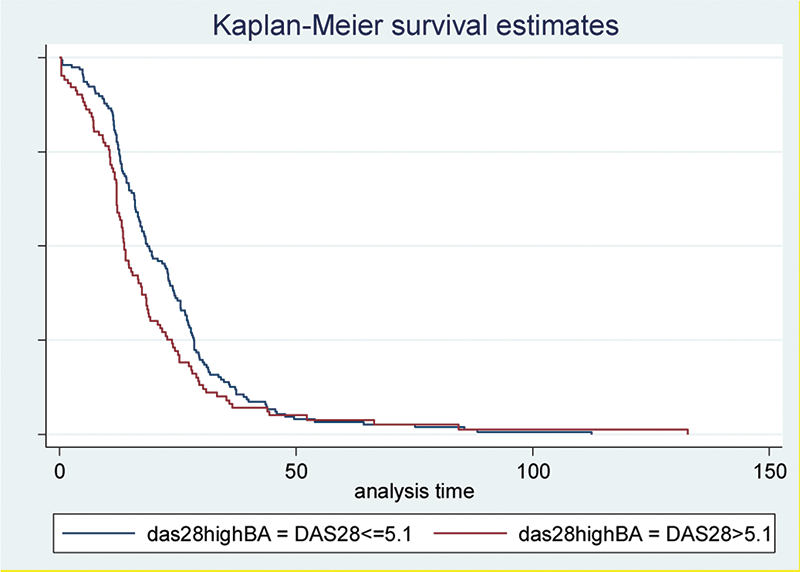
Conclusion: In Mexican RA patients registered in BIOBADAMEX, we found that baseline high disease activity is associated with the discontinuation of bDMARDs. Further longitudinal analyses will be performed including more patients to assess retention rate of specific bDMARDs and identify predictive variables of discontinuation in Mexican population.
REFERENCES:
[1]Lauper K, Finckh A. Predictive factors of treatment persistence in rheumatoid arthritis. Joint Bone Spine. 2020 Dec;87(6):531-534.
Disclosure of Interests: VIJAYA RIVERA TERAN: None declared, David Vega-Morales: None declared, Sandra Sicsik: None declared, Fedra Irazoque-Palazuelos: None declared, Miguel A Saavedra: None declared, Julio Cesar Casasola: None declared, Sandra Carrilo: None declared, Angélica Peña: None declared, Angel Castillo Ortiz: None declared, Omar Eloy Muñoz-Monroy: None declared, Sergio Duran Barragan: None declared, Azucena Ramos: None declared, Luis Francisco Valdés Corona: None declared, Estefanía Torres Valdéz: None declared, Aleni Paz: None declared, ERICK ADRIAN ZAMORA-TEHOZOL: None declared, Daniel Xavier Xibille Friedmann: None declared, Francisco Guerrero: None declared, Natalia Santana: None declared, Miguel Vazquez: None declared, Claudia Zepeda: None declared, Melanea Rivera: None declared, Kitzia Alvarado: None declared, Deshire Alpizar-Rodriguez Consultant of: Scientific advisor for GSK, unrelated to this study., Employee of: Scientific advisor for GSK, unrelated to this study.