

Background: AVT02 is an investigational biosimilar to adalimumab. It is approved in Europe, Canada, and the UK. It is not approved by the US Food and Drug Administration (FDA).
Objectives: To evaluate the pharmacokinetic similarity of 100 mg/mL AVT02, an investigational biosimilar of adalimumab, when administered either via a pre-filled syringe, or with a newly developed autoinjector in healthy adult subjects.
Methods: This was a Phase 1, randomized, open-label, parallel-group study in which 207 healthy adult subjects were randomized in a 1:1 ratio to receive 100 mg/mL AVT02 either via a pre-filled syringe, or with an autoinjector, stratified by body weight. Subjects received a single subcutaneous 40 mg dose on Day 1. Pharmacokinetics, immunogenicity, local injection site reactions, and adverse events were assessed prior to, and up to 64 days after, study drug administration.
Results: The results observed supported the assessment of pharmacokinetic similarity of investigational AVT02 administered by pre-filled syringe or with an autoinjector. The 90% CIs for the ratios of geometric least square means for the primary pharmacokinetic parameters C
max
, AUC
0-t
, and AUC
0-∞
were contained within prespecified margins 80% and 125%, based on an analysis of variance model with treatment as a fixed effect. The mean serum concentration-time profile of adalimumab by treatment group is shown in
Mean Serum Concentration-Time Profile of Adalimumab by Treatment Group on Semilogarithmic Scale (Pharmacokinetic Population)
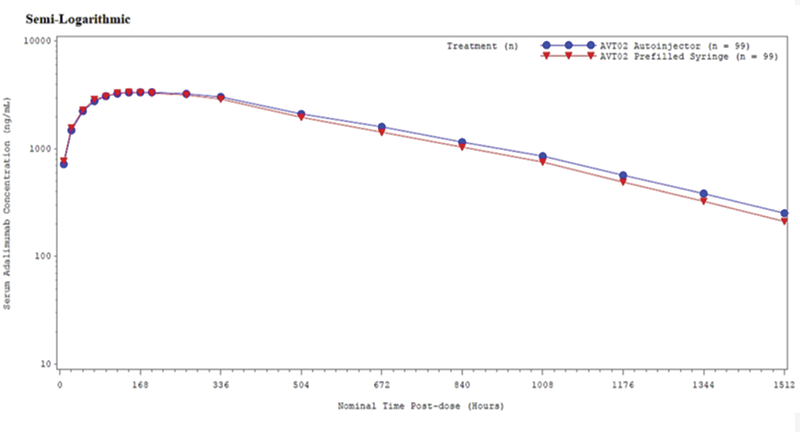
Binding anti-drug antibodies were detectable at the end of study visit on Day 64 in 100% and 97.0% of subjects in the pre-filled syringe administration and the autoinjector groups, respectively. Of those subjects positive for anti-drug antibodies, 85.7% and 86.5% further tested positive for neutralizing antibodies in the pre-filled syringe administration and autoinjector groups, respectively. The frequency of local administration site reactions was 11.8% overall and similar between treatment groups. The most frequently reported treatment-emergent adverse events in both treatment groups were under the SOC: Infections and infestations (56.0% in the AVT02-pre-filled syringe group and 45.2% in the AVT02-autoinjector group). The safety profiles were generally similar between treatment groups.
Conclusion: The results observed supported the assessment of pharmacokinetic similarity between the pre-filled syringe and autoinjector delivery systems after a single subcutaneous 40 mg dose. The autoinjector delivery system was generally well tolerated in healthy subjects, with a safety and immunogenicity profile similar to that observed with 100 mg/mL AVT02 administered using a pre-filled syringe.
ClinicalTrials.gov Identifier: NCT03983876
Disclosure of Interests: Christopher Wynne: None declared, Heimo Stroissnig Employee of: Alvotech, Roshan Dias Employee of: Alvotech, Joanna Sobierska Employee of: Alvotech, Eric Guenzi Employee of: Alvotech, Hendrik Otto Employee of: Alvotech, Abid Sattar Employee of: Alvotech, Halimu N. Haliduola Employee of: Alvotech, Elin Edwald Employee of: Alvotech, Fausto Berti Employee of: Alvotech