

Background: Pausing methotrexate (MTX) for two to four weeks, improved immunogenicity of influenza vaccination in patients with rheumatoid arthritis (RA), albeit a risk of disease flare (1). This guided the framing of guidelines on MTX withdrawal for COVID-19 vaccination (2). However, evidence for MTX withdrawal for COVID-19 vaccination is limited to observational studies only.
Objectives: To compare the efficacy and safety of holding MTX after each (MIVAC 1) and only after the second dose (MIVAC II) of the ChAdOx1 vaccine versus continuation of MTX in two randomized controlled trials (RCTs).
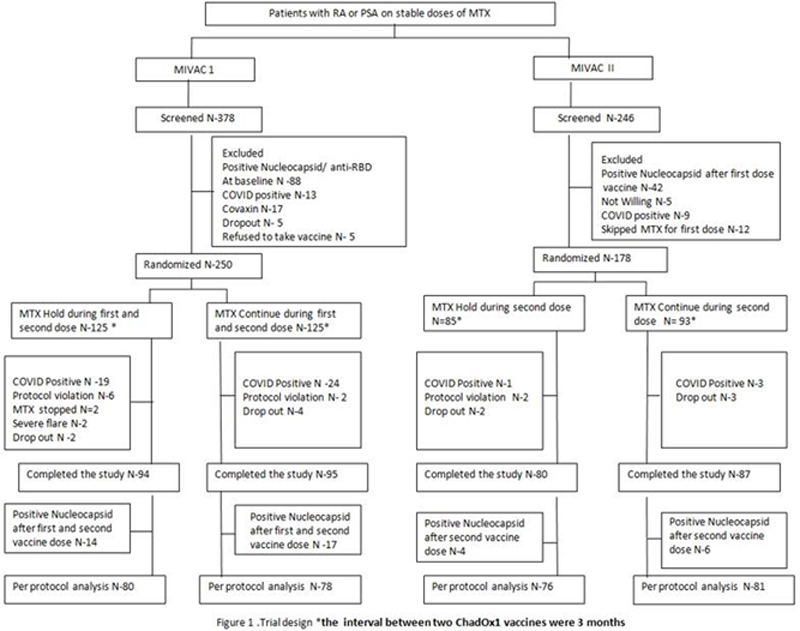
Methods: Two single centre, investigator-blinded, RCTs were conducted in patients with RA or Psoriatic arthritis (PsA) on stable doses of MTX without prior COVID-19 (CTRI reg. no. MIVAC I: CTRI/2021/07/03463 & MIVAC II: CTRI/2021/07/035307). In MIVAC I, unvaccinated patients were randomised (1:1) to hold or continue MTX for two weeks after each dose of the vaccine. MIVAC II included patients who had continued MTX during the first dose of ChAdOx1 and were randomised (1:1) to hold or continue MTX for 2 weeks after the second vaccine dose. The primary outcome for both the trials was the anti-Receptor Binding Domain (RBD) antibody titres measured four weeks after the second vaccine dose (per protocol analysis). Secondary outcome was the flare rate, defined as an increase in disease activity scores (DAS28/cDAPSA) or physician intent to hike DMARDs.
Results: 250 patients were randomized for MIVAC 1 and 178 for MIVAC II and after due exclusions, 158 and 157 were eligible for analysis respectively (
Baseline demographics and key results
| Variable | MIVAC I | MIVAC II | ||||
|---|---|---|---|---|---|---|
| MTX Hold | MTX Continue | P | MTX Hold | MTX Continue | P value | |
| N=80 | N=78 | value | N=76 | N=81 | ||
| Age† | 48 (38-53.3) | 49 (39-59) | 0.19 | 53 (42.3-59) | 53(50-62) | 0.14 |
| Female (%) ‡ | 73 (91.3) | 75 (96.2) | 0.33 | 65 (85.5) | 70 (86.4) | >0.99 |
| RA (%) ‡ | 69(86.3) | 69 (93.2) | 70 (85.6) | 80 (87.7) | ||
| PsA (%) ‡ | 11(13.8) | 6 (8.1) | 0.31 | 6 (7.9) | 1 (1.2) | 0.057 |
| DAS28† | 2.7 (2.4-3.2) | 2.6 (2-3.3) | 0.6 | 2.7(2.3-3.4) | 2.8 (2.1-3.5) | 0.78 |
| cDAPSA † | 2(3-4.5) | 2.5(1.3-3.8) | 0.46 | 3(2.8-3) | 3 | 0.15 |
| Prednisolone (%) ‡ | 29 (36.3) | 23(31.1) | 0.4 | 24(31.6) | 26 (32.1) | >0.99 |
| MTX mg/week† | 17.5 (10-25) | 15 (10-20) | 0.057 | 15 (9.4-25) | 17.5(7.5-25) | 0.92 |
| Anti- RBD antibody titres post second dose (IU/mL) † | 2484 (1050-4388.8) | 1147.5 (433.5-2360.3) | <0.001 | 2553.5 (1792.5-4823.8) | 990.5 (356.1-2252.5) | <0.001 |
| Flare (N%) ‡ | ||||||
| Post first dose | 20 (25) | 6 (8) | 0.005 | NA | NA | |
| Post second dose | 19 (23.8) | 10(13.3) | 0.1 | 9 (11.8) | 4 (7.9) | 0.15 |
All analysis as per protocol population.
†Median (interquartile range): Mann Whitney U test.
‡ N (%): Fisher Exact test
. Bolded if p<0.05.
Conclusion: Holding MTX after both the doses or only after the second dose of ChAdOx1 yields higher anti-RBD antibody titres as compared to continuing MTX. Comparing across the trials, holding MTX only after the second dose appears to be non-inferior to holding MTX after both doses of the vaccine with a lesser risk of flare.
REFERENCES:
[1]Park JK et al. Clin Rheumatol. 2020 Feb; 39(2):375-379.
[2]Curtis JR, et al. Arthritis & Rheumatology. 2021 Oct;73(10): e60-75.
Acknowledgements: Acknowledgments to all participating investigators, patients and their families
Disclosure of Interests: Anu Sreekanth: None declared, Teny Skaria: None declared, Sneha Joseph: None declared, Rashwith Umesh: None declared, Manju Mohanan: None declared, Aby Paul: None declared, Sakir Ahmed Speakers bureau: Sakir Ahmed had received honorarium as speaker from Pfizer, Dr Reddy’s, Cipla, and Novartis unrelated to this Comment, Pankti Mehta: None declared, Seena Oomen: None declared, Janet Benny: None declared, Justin George: None declared, Anagha Paulose: None declared, K Narayanan: None declared, Sanjana Joseph: None declared, Anuroopa Vijayan: None declared, Kaveri Nalianda: None declared, Padmanabha Shenoy: None declared