

Background: Fatigue, one of the top 3 patient (pt)-reported symptoms of psoriatic arthritis (PsA) and a recent PsA outcome domain, 1 causes impaired health-related quality-of-life, diminished productivity, and disability. 1-3 Although the origins of fatigue are multifactorial, inflammation is hypothesized to play an important role. 4 In pts with active PsA, treatment with guselkumab (GUS) led to clinically meaningful and sustained improvements in fatigue through 1 year in DISCOVER-1 (D1) and DISCOVER-2 (D2). 5
Objectives: To identify 1) factors associated with fatigue and 2) factors associated with change in fatigue among pts with PsA treated with GUS.
Methods: In the Phase 3 D1 (N=381, biologic-naïve and tumor necrosis factor inhibitor-experienced) and D2 (N=739, biologic-naïve) studies, pts with active PsA despite standard therapies and/or biologic disease-modifying antirheumatic drugs were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or placebo (PBO) with crossover to GUS 100 mg Q4W at W24. The pt-reported Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) scale measured fatigue (scored 0-52). In these post-hoc analyses of D1 and D2 pts, a principal component analysis (PCA) was performed using W0 data to identify the underlying baseline factors associated with fatigue. Additionally, linear regression analyses were performed to identify covariates associated with change in fatigue from W0 to W24.
Results: In 1120 pts (mean age 47 yrs, mean disease duration 5.9 yrs, 48% female), mean FACIT-Fatigue scores at baseline ranged from 29.1 to 31.4 (vs 43.6 for the general US population).
5
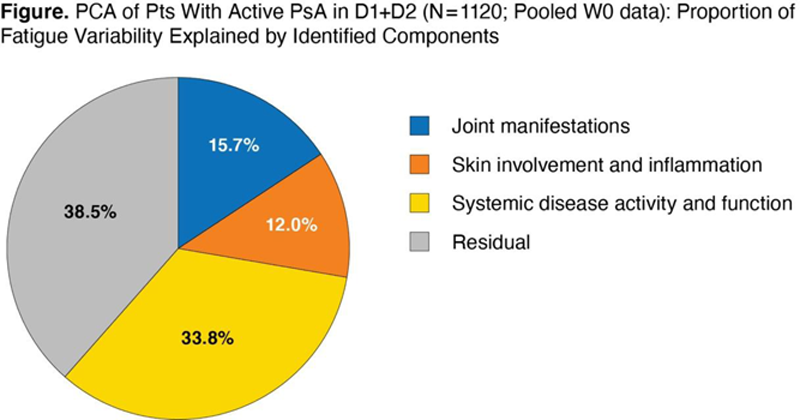
PCA showed that 62% of the variability in fatigue could be explained by 3 components (
PCA of Pts With Active PsA in D1+D2 (N=1120; Pooled W0 data): Factor Loading Estimates by Covariates
| Component1 Systemic Disease Activity and Function | Component 2 Joint Manifestations | Component 3 Skin Involvement and Inflammation | |
|---|---|---|---|
| PsA disease duration, yr | 0.10 | 0.14 | 0.25 |
| PASI total score (0-72) | 0.22 | 0.23 | 0.74 |
| CRP, mg/dL | 0.36 | -0.13 | 0.55 |
| HAQ-DI score (0-3) | 0.73 | -0.09 | -0.19 |
| Pain (0-10 VAS) | 0.83 | -0.35 | -0.13 |
| PtGDA (0-10 VAS) | 0.82 | -0.36 | -0.16 |
| Physician global assessment of disease activity (0-10 VAS) | 0.65 | -0.18 | 0.23 |
| SJC (0-66) | 0.50 | 0.74 | -0.12 |
| TJC (0-68) | 0.54 | 0.70 | -0.18 |
VAS=Visual Analog Scale.
Conclusion: Among pts with PsA, measures of systemic disease activity and function, followed by joint manifestations, and skin involvement/inflammation accounted for 62% of the variability in fatigue. The large residual effect (38%) that was unexplained by the current model suggests the need for further research to identify additional factors (eg, distinct molecular pathways) contributing to the fatigue reported by PsA pts.
REFERENCES:
[1]Leung YY, et al. J Rheumatol (Suppl ). 2020;96:46-9.
[2]Gudu T, et al. Joint Bone Spine . 2016;83:439-43.
[3]Husted JA, et al. Ann Rheum Dis . 2009;68:1553-8.
[4]Krajewska-Włodarczyk M, et al. Reumatologia . 2017;55:125-30.
[5]Rahman P, et al. Arthritis Res Ther . 2021;23:190.
Disclosure of Interests: Proton Rahman Consultant of: AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer, and UCB, Grant/research support from: Janssen and Novartis, Philip J Mease Speakers bureau: AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, and UCB, Consultant of: AbbVie, Aclaris, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GSK, Inmagene, Janssen, Novartis, Pfizer, Sun Pharma, and UCB, Grant/research support from: AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, Sun Pharma, and UCB, Atul Deodhar Speakers bureau: AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, Consultant of: AbbVie, Amgen, Aurinia, Bristol Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, MoonLake, Novartis, Pfizer, and UCB, Grant/research support from: AbbVie, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, and UCB, Arthur Kavanaugh Consultant of: AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Genentech, Janssen, Merck, Novartis, Pfizer and UCB, Soumya D Chakravarty Shareholder of: Johnson & Johnson, Employee of: Janssen Scientific Affairs, LLC, Alexa Kollmeier Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Yan Liu Shareholder of: 3 Johnson & Johnson, Employee of: Janssen Research & Development, LLC, May Shawi Shareholder of: Johnson & Johnson, Employee of: Janssen Pharmaceutical Companies of Johnson & Johnson, Chenglong Han Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC.