

Background: Olokizumab (OKZ) is an interleukin-6-inhibitor for treatment of Rheumatoid Arthritis (RA). In these analyses, we present patient reported outcomes (PROs) reported by MTX-IR patients with moderate to severely active RA treated with OKZ vs adalimumab (ADA) or placebo in a phase 3 randomized controlled trial (RCT) (ClinicalTrials.gov number, NCT02760407).
Objectives: To assess the effect of OKZ treatment compared with placebo and ADA in patient global assessment of disease activity (PtGA), pain, physical function (HAQ-DI), fatigue (FACIT-F) and health related quality of life (SF-36 physical (PCS) and mental (MCS) component summary and domain scores) and work participation (WPS-RA) at week 12.
Methods: 1648 patients receiving MTX were randomized to receive SQ injections: 1) OKZ 64 mg every 2 weeks (q2w, n=464), 2) OKZ 64 mg q4w (n=479), 3) ADA 40 mg q2w (n=462) and 4) placebo q2w (n=243). At week 14, non-responders: subjects without ≥ 20% improvements in both swollen and tender joint counts, added rescue medication (sulfasalazine and/or hydroxychloroquine) to study treatment. Between groups differences in least-squares mean (LSM) changes from baseline were analyzed.
Results: At week 12, treatment with both OKZ doses and ADA resulted in statistically greater LSM changes from baseline than placebo across all PROs, including 7 of 8 domains of SF-36 with exception of role emotional (
Mean baseline PROs and LSM changes to week 12
| Baseline, mean (standard deviation) | 12 weeks LSM changes (standard error) | |||||||
|---|---|---|---|---|---|---|---|---|
| OKZ q2w, N=464 | OKZ q4w, N=479 | ADA q2w, N=462 | Placebo, N=243 | OKZ q2w, N=464 | OKZ q4w, N=479 | ADA q2w, N=462 | Placebo, N=243 | |
| PtGA-VAS | 67.5(20.2) | 66.8(20.9) | 66.7(21.0) | 67.4(20.0) | -29.7(1.1)# | -29.5(1.0)# | -31.6(1.1)# | -21.0(1.5) |
| Pain-VAS | 68.4(20.6) | 67.1(21.0) | 66.8(21.5) | 66.5(20.7) | -31.8(1.1)# | -31.7(1.1)# | -32.7(1.1)# | -21.3(1.6) |
| HAQ-DI* | 1.7(0.58) | 1.7(0.60) | 1.7(0.57) | 1.7(0.62) | -0.6(0.03)# | -0.6(0.03)# | -0.6(0.03)# | -0.4(0.04) |
| Comparison vs. ADA LSM difference [97.5% CI] | -0.03 [-0.12;0.05] | 0.00 [-0.08;0.08] | ||||||
| SF-36 PCS | 31.8(7.0) | 31.6(7.2) | 31.4(7.4) | 31.9(7.5) | 8.1(0.4)# | 7.8(0.4)# | 8.1(0.4)# | 4.9(0.5) |
| SF-36 MCS | 42.9(11.4) | 43.50(11.3) | 44.1(11.4) | 43.1(11.0) | 5.1(0.4) † | 4.9(0.4) † | 5.0(0.4) † | 3.1(0.6) |
| FACIT-F | 26.7(10.7) | 27.3(10.4) | 27.4(11.3) | 27.3(10.2) | 8.4(0.4)# | 8.1(0.4 )ⱡ | 8.9(0.4)# | 5.2(0.6) |
Footnotes: LSM difference (SE) 97.5% CI by ANCOVA. NRS imputation.
*, secondary endpoint; †p≤0.05, ⱡp<0.01, #p<0.001 vs placebo; VAS (mm).
SF-36 domain changes from baseline to week 12. *p≤0.05, **p<0.01, ***p<0.001 ADA vs placebo; *p≤0.05, **p<0.01, ***p<0.001 OKZ q2w vs placebo; *p≤0.05, **p<0.01, ***p<0.001 OKZ q4w vs placebo. AGNorms, age- and gender-matched; BL, baseline.
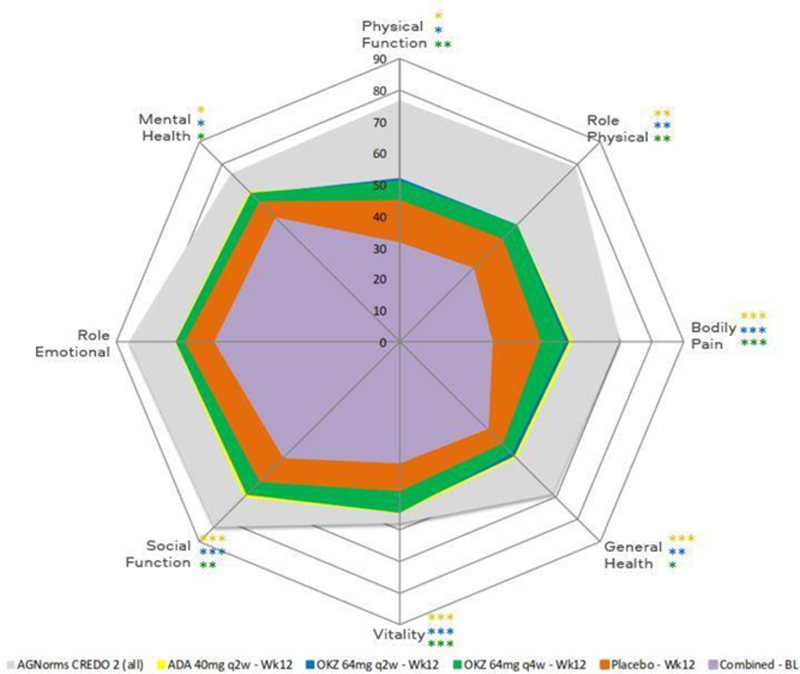
Conclusion: Treatment with both doses of OKZ resulted in similar, statistically significant improvements across PROs vs placebo in MTX-IR patients with moderate to severely active RA, comparable to ADA, that were clinically meaningful.
Acknowledgements: R-Pharm funded this study; contributed to its design; participated in data collection, analysis, and interpretation of the data; and in the writing, review, and approval of the abstract. No honoraria or payments were made for authorship.
Disclosure of Interests: Vibeke Strand Consultant of: Abbvie, Amgen, Arena, AstraZeneca, Bayer, BMS, Boehringer, Ingelheim, Chemocentryx, Celltrion, Galapagos, Genentech/Roche, Gilead, GSK, Horizon, Inmedix, Janssen, Kiniksa, Lilly, Novartis, Pfizer, Regeneron, Rheos, R-Pharm, Samsung, Sandoz, Sanofi, Scipher, Servier, Setpoint, Sorrento, Spherix, UCB, Ernest Choy Consultant of: Abbvie, Amgen, Bristol Myer Squibbs, Chugai Pharma, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, Regeneron, RPharm, Roche, Sanofi, and UCB., Grant/research support from: Bio-Cancer, Biogen, Novartis, Pfizer, Roche, Sanofi and UCB, Evgeny Nasonov Consultant of: AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, Tatiana Lisitsyna: None declared, Alexander Lila Consultant of: Abbvie, Amgen, Bayer, Biotechnos, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, RPharm, Roche, Sanofi, Stada, Viatris and UCB, Sofia Kuzkina Employee of: R-Pharm, Mikhail Samsonov Employee of: R-Pharm, Eugen Feist Consultant of: Abbvie, Eli Lilly, Galapagos, Medac, Novartis, Sanofi, Sobi, R-Pharm, Grant/research support from: Eli Lilly, Novartis, Pfizer.