

Background: Bone erosion in rheumatoid arthritis (RA) is partly caused by excessive activation of osteoclasts[1]. Osteoclasts can be derived from RA synovium and their differentiation can be inhibited by OPG, a decoy receptor of the osteoclastogenesis promoting cytokine RANKL[2,3]. Fibroblast-like synoviocytes (FLSs) are a main type of stromal cells in the synovium that can secret OPG[4]. The OPG secretion by FLSs can be modulated by various cytokines[5]. Interleukin (IL)-13 is a cytokine rich in early RA synovial fluid and it decreases as RA progresses[6]. IL-13 was reported to alleviate bone erosion in RA mouse models[7]. However, how IL-13 reduces bone destruction remains unclear.
Objectives: To investigate if IL-13 can inhibit osteoclast differentiation by up-regulating OPG in FLSs of RA patients (RA-FLSs), thus reduces bone erosion in RA.
Methods: FLSs were isolated from the synovium tissue of RA patients with informed consent who underwent joint replacement surgery (Ethics No. 2021-544-01). OPG and RANKL expression by RA-FLSs were evaluated by qRT-PCR. OPG secretion was determined by ELISA. Western blot was performed to analyze OPG expression and the activation of STAT6 pathway. IL-13 and OPG siRNA pre-treated RA-FLSs conditioned medium were used in osteoclast induction to test if IL-13 can inhibit osteoclastogenesis by up-regulating OPG in RA-FLSs. Micro-CT scanning and immunofluorescence were done to determine if IL-13 can induce OPG expression and alleviate bone erosion in vivo.
Results: IL-13 can significantly upregulate OPG expression and secretion by RA-FLSs. Meanwhile, RANKL/OPG ratio was downregulated (
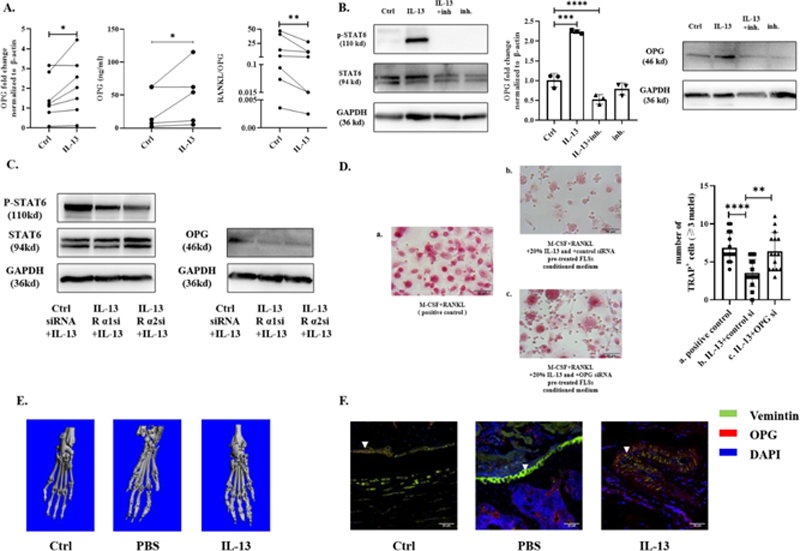
Conclusion: IL-13 can inhibit osteoclastogenesis by up-regulating OPG in RA-FLSs through IL-13 receptors via STAT6 pathway, thus ameliorate bone erosion in RA.
REFERENCES:
[1]Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol 2012;8(11):656-64.
[2]Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front Immunol 2014;5:511.
[3]Gravallese EM, Harada Y, Wang JT, et al. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol 1998;152(4):943-51.
[4]Ziolkowska M, Kurowska M, Radzikowska A, et al. High levels of osteoprotegerin and soluble receptor activator of nuclear factor kappa B ligand in serum of rheumatoid arthritis patients and their normalization after anti-tumor necrosis factor alpha treatment. Arthritis Rheum 2002;46(7):1744-53.
[5]Tunyogi-Csapo M, Kis-Toth K, Radacs M, et al. Cytokine-controlled RANKL and osteoprotegerin expression by human and mouse synovial fibroblasts: fibroblast-mediated pathologic bone resorption. Arthritis Rheum 2008;58(8):2397-408.
[6]Raza K, Falciani F, Curnow SJ, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther 2005;7(4):R784-95.
[7]Woods JM, Amin MA, Katschke KJ, Jr., et al. Interleukin-13 gene therapy reduces inflammation, vascularization, and bony destruction in rat adjuvant-induced arthritis. Hum Gene Ther 2002;13(3):381-93.
Acknowledgements: This study was supported by the National Natural Science Foundation of China (grant number 81671608). We would like to thank Prof. Zhihong Xu and Dr. Minghui Sun for the assistance in synovial tissue collection, and all the patients and participants for their support for our study.
Disclosure of Interests: None declared.