

Background: Recently, the core domains of the 20-years old core outcome set for ankylosing spondylitis were updated. 1 The next step is to define the measurement core set, which includes at least one instrument for each domain.
Objectives: To define the instruments for the ASAS-OMERACT core outcome set for axial spondyloarthritis (axSpA).
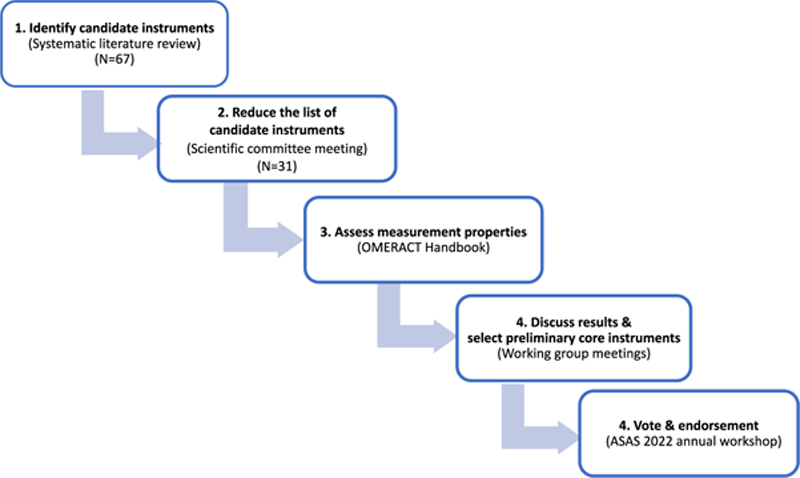
Methods: The scientific committee invited an international working group representing all key stakeholders (patients, rheumatologists, health professionals and pharmaceutical industry). The instrument selection process is presented in
Development process to determine the core measurement set
Results: The updated core measurement set for axSpA is shown in
Updated core measurement set for axial spondyloarthritis.
| Instruments mandatory for all trials | |
|---|---|
| Domain | Instrument |
| Disease activity | ASDAS |
| Patient global assessment of disease activity (NRS) | |
| Pain | NRS total back pain (BASDAI Q2) |
| Morning stiffness | Severity and duration (BASDAI (Q5+Q6)/2)) |
| Fatigue | NRS fatigue (BASDAI Q1) |
| Physical function | BASFI |
| Overall functioning & health | ASAS Health Index |
| Additional instruments mandatory for disease modifying drugs trials | |
| Disease activity | SPARCC MRI-SIJ* |
| SPARCC MRI-spine* | |
| Extra-musculoskeletal manifestations | uveitis (ASAS CRF) 2 |
| psoriasis (ASAS CRF) 2 | |
| inflammatory bowel disease (ASAS CRF) 2 | |
| Peripheral manifestations | 44 Swollen joint count |
| MASES | |
| Dactylitis count (ASAS CRF) 2 | |
| Structural damage | mSASSS* |
*Needs to be assessed at least once in a disease modifying drug programme; 2 Dougados M, et al. Ann Rheum Dis 2012;71(6):1103-04. ASDAS: Ankylosing Spondylitis Disease Activity Score; NRS: Numerical Rate Scale; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; Q: question; BASFI: Bath Ankylosing Spondylitis Functional Index; SPARCC: SpondyloArthritis Research Consortium of Canada Scoring System; MRI: Magnetic Resonance Imaging; SIJ: Sacroiliac Joint; CRF: Case Report Form; MASES: Maastricht Ankylosing Spondylitis Enthesitis Score; mSASSS: modified Stoke Ankylosing Spondylitis Spinal Score.
Conclusion: The previous core measurement set has been updated and endorsed by ASAS for the use in all axSpA trials.
REFERENCES:
[1]Navarro-Compán V, et al. Semin Arthritis Rheum 2021;51(6):1342-49.
[2]Dougados M, et al. Annals of the Rheumatic Diseases 2012;71(6):1103-04.
Acknowledgements: The ASAS axSpA core measurement set working group:
Désirée van der Heijde
Victoria Navarro Compán
Annelies Boonen
Philip Mease
Anne Boel
Uta Kiltz
Robert Landewé
Maxime Dougados
Xenofon Baraliakos
Wilson Bautista
Pravina Chiowchanwisawakit
Yu Heng Kwan
Lianne Gensler
Bassel El-Zorkany
Karl Gaffney
Nigel Haroon
Pedro Machado
Walter Maksymowych
Anna Molto
Denis Poddubnyy
Mikhail Protopopov
Sofia Ramiro
Salima van Weely
Marco Garrido Cumbrera
Natasha de Peyrecave
Lara Fallon
In-Ho Song
Hanne Dagfinrud
The Assessment of Spondyloarthritis international Society (ASAS) supported Anne Boel and Victoria Navarro-Compán financially to update the core outcome set.
Disclosure of Interests: Victoria Navarro-Compán Speakers bureau: AbbVie, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB Pharma, Consultant of: AbbVie, Eli Lilly, MSD, Novartis, Pfizer, UCB Pharma; Research grants from AbbVie and Novartis, Grant/research support from: AbbVie and Novartis, Anne Boel: None declared, Annelies Boonen Speakers bureau: Abbvie / Galapagos, Consultant of: Galapagos, Grant/research support from: AbbVie, Philip J Mease Speakers bureau: Abbvie, Janssen, Lilly, Novartis, Pfizer, UCB, Consultant of: Abbvie, Aclaris, Amgen, Bristol Myers, Boehringer-Ingelheim, Galapagos, Gilead, GlaxoSmithKline, Inmagene, Janssen, Lilly, Novartis, Pfizer, SUN Pharma, UCB, Grant/research support from: Abbvie, Bristol Myers, Gilead, Inmagene, Janssen, Lilly, Novartis, Pfizer, UCB, Maxime Dougados: None declared, Uta Kiltz Consultant of: AbbVie, Chugai, Eli Lilly, Fresenius, Hexal, Janssen, MSD, Novartis, onkowissen.de, Pfizer, Roche and UCB, Grant/research support from: Abbvie, Amgen, Biogen, Hexal, Novartis und Pfizer, Robert B.M. Landewé Consultant of: AbbVie, BMS, Galapagos, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, UCB, Désirée van der Heijde Consultant of: AbbVie, Bayer, BMS, Cyxone, Eisai, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Lilly, Novartis, Pfizer, UCB Pharma, Employee of: Director of Imaging Rheumatology bv.