

Background: Psoriatic arthritis (PsA) is a chronic, immune-mediated inflammatory disease with heterogenous musculoskeletal manifestation (arthritis, spondylitis, enthesitis, dactylitis) and extra-musculoskeletal manifestation (skin and nail psoriasis). In addition, PsA is commonly associated with comorbidities such as metabolic syndrome and cardiovascular diseases where IL-17 is a key driver of this disease.
Izokibep is a unique IL-17A inhibitor with extraordinary potency and small molecular size designed to overcome the limitations of monoclonal antibodies such as poor tissue distribution.
Here, we report 16-week phase 2 results in patients with active PsA.
Objectives: To assess efficacy, safety, pharmacokinetics and immunogenicity of izokibep versus placebo.
Methods: This is a prospective, multicenter, randomized, double-blind, placebo-controlled, parallel-groups, dose-finding trial studying bi-weekly 80 mg or 40 mg izokibep administered subcutaneously versus placebo until Week 16 (Period 1) and dose-controlled treatment until Week 46 (Period 2). PsA patients had to have ≥3 swollen and ≥3 tender joints of the 66/68 joint count, and an inadequate response to previous NSAIDs, csDMARDs or TNF inhibitor therapy. The primary endpoint was to evaluate ACR50 responses of 80 mg bi-weekly versus placebo at Week 16. Key secondary endpoints were ACR20/70, MDA, DAS28, DAPSA, SPARCC, LDI, PASI as well as tolerability and safety. Efficacy outcome measures were assessed in all patients who received at least one dose of study treatment. This trial is registered with ClinicalTrials.gov, NCT04713072.
Results: 135 patients were randomized and treated between June 2020 and July 2021 in 22 European sites located in Austria, Belgium, Czech Republic, Germany, Hungary, Poland and Spain.
At baseline, patients had a mean age of 48.5 (SD 12.0) years, a mean BMI of 29.0 (SD 4.8) kg/m 2 , a mean swollen joint count (SJC) of 9.9 (SD 6.6), and a mean tender joint count (TJC) of 16.7 (SD 10.4). The mean PsA disease duration was 7.1 (SD 7.8) years. 13% failed previous TNF inhibitor treatment and 80% received a concomitant csDMARD.
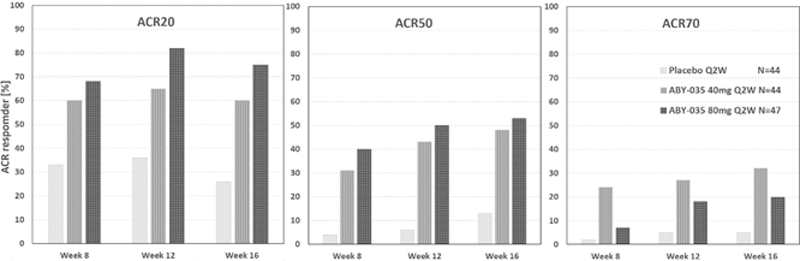
At Week 16, the confirmatory primary endpoint ACR50 response rate was met (p=0.0003). ACR50 response rate was 52% in the 80 mg group, 48% in the 40 mg and 13% in the placebo group. The ACR20/50/70 response rates up to Week 16 by treatment group are presented in
ACR20/50/70 response rates
SJC and TJC rapidly decreased with active treatment as indicated in
SJC and TJC by visit until Week 16
| Mean SJC (SD ) | Mean TJC (SD ) | |||||
|---|---|---|---|---|---|---|
| Study Week | Placebo Q2W N=44 | 40 mg Q2W N=44 | 80 mg Q2W N=47 | Placebo Q2W N=44 | 40 mg Q2W N=44 | 80 mg Q2W N=47 |
| BL | 9.2 (6.4) | 10.1 (7.0) | 10.4 (6.4) | 16.4 (11.3) | 16.7 (10.3) | 17.0 (9.7) |
| 2 | 8.4 (6.1) | 7.3 (6.5) | 7.6 (7.6) | 14.9 (10.0) | 13.3 (9.5) | 13.8 (10.7) |
| 4 | 7.7 (7.7) | 6.0 (7.0) | 5.6 (6.2) | 14.1 (11.8) | 12.5 (11.6) | 11.2 (9.2) |
| 8 | 6.0 (6.2) | 3.5 (4.1) | 3.7 (4.7) | 10.5 (7.5) | 9.0 (10.5) | 7.4 (7.2) |
| 12 | 5.1 (5.2) | 2.6 (3.4) | 2.3 (3.4) | 10.9 (8.7) | 8.1 (8.9) | 6.0 (6.7) |
| 16 | 5.0 (5.7) | 2.4 (3.7) | 1.7 (2.7) | 10.7 (9.1) | 7.1 (7.7) | 5.6 (6.8) |
There was a dose-response relationship and a fast onset of response.
No serious or severe adverse events occurred during Period 1. The three most frequently affected System Organ Classes (SOCs) were SOC General disorders and administration site conditions comprising mainly mild injection site reactions or erythema followed by SOC Infections and infestations and SOC Metabolism and nutrition disorders. One mild, transient vulvovaginal Candida infection with active treatment was reported. Apart from injection site reactions there were no apparent differences in the occurrence of adverse events between active and placebo patients.
Conclusion: In this phase 2 study, izokibep showed a dose-dependent high degree of efficacy in patients with active PsA having failed previous treatment. Overall, izokibep was well tolerated.
These data strongly support further clinical development.
REFERENCES:
None
Acknowledgements: Affibody AB, Sweden, and ACELYRIN Inc, USA, funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of this publication. All authors participated in the drafting, review, and approval of this publication.
Disclosure of Interests: Frank Behrens Shareholder of: Pfizer, Sanofi, GlaxoSmithKline, Gilead Sciences, Inc., Novartis, Speakers bureau: Amgen, Horizon, Lilly, Novartis, Pfizer, Sanofi, Genzyme, Flexion, AbbVie, Consultant of: AbbVie, Boehringer Ingelheim, Flexion, Janssen, Pfizer, Sanofi, Regeneron, SUN Pharma Advanced Research, Gilead Sciences, Inc., Grant/research support from: Pfizer, Janssen, Chugai, Celgene, Roche, Alan Kivitz, Peter C. Taylor Consultant of: AbbVie, Biogen, Bristol Myers Squibb, Fresenius, Galapagos, Gilead, GlaxoSmithKline, Janssen, Lilly, Nordic Pharma, Pfizer, Roche, Sanofi, and UCB, Grant/research support from: Celgene and Galapagos, Dieter Wetzel Consultant of: Acino, Affibody, Biotest, Cheplapharm, CSL Behring, Mundipharma, Roche, Sandoz, Temmler, Nikolai C Brun Employee of: Affibody AB, Jan Brandt-Juergens Speakers bureau: Abbvie, Pfizer, Roche, Sanofi-Aventis, Novartis, Lilly, MSD, UCB, BMS, Janssen, Medac, Gilead, Affibody, Paid instructor for: Abbvie, Pfizer, Roche, Sanofi-Aventis, Novartis, Lilly, MSD, UCB, BMS, Janssen, Medac, Gilead, Affibody, Edit Drescher: None declared, Eva Dokoupilova: None declared, Anna Rowińska-Osuch: None declared, Nadia Abdel-Kader Martin Speakers bureau: Pfizer in 2011, Kurt de Vlam Speakers bureau: AbbVie, Amgen, Eli Lilly, Novartis, UCB, Paid instructor for: Amgen, Galapagos, UCB, Consultant of: Eli Lilly, Johnson &Johnson, Novartis Galapagos, UCB, Grant/research support from: Celgene