

Background: The recently presented “ORAL Surveillance Study” has suggested increased risk of serious adverse events (AEs) with tofacitinib, a JAK-inhibitor (JAKi), compared to a comparator TNF-inhibitor (TNFi). Currently, there is limited real world evidence for the tolerability and safety of JAKi (1).
Objectives: To assess the safety of JAKi compared to other biologic agents in rheumatoid arthritis (RA) patients in a real-world population, by evaluating treatment discontinuation for AEs.
Methods: Pooled patient database from 16 national RA registries from across Europe, Québec (Canada), Turkey, and Israel were used. Treatment discontinuation due to AEs by treatment groups, JAKi versus (vs) TNFi and JAKi vs bDMARDs with other modes of action (OMA), were compared as an overall measure of tolerability and safety of JAKi. Standard descriptive statistics were used for baseline characteristics. We plotted unadjusted cumulative incidence, then the cause-specific Cox model was used to account for competing risks, and to obtain association between covariates and the instantaneous hazard rate for AEs. Variables listed in
Baseline characteristics of the study population
| JAKi 1 (BARI, FILGO,TOFA,UPA) | OMA 2 (ABA, ANAK, SARI, TOCI) | TNFi 3 (ADA, CERT, ETAN, GOL, INFL) | |
|---|---|---|---|
| n = 9208 | n = 16737 | n = 64533 | |
| Treatment duration* (yrs ) | 0.7 [0.2, 1.7] | 1.1 [0.4, 2.8] | 1.5 [0.5, 3.9] |
| Age | 57.5 | 56.8 | 53.2 |
| Female (% ) | 81.3 | 80.7 | 73.2 |
| Disease duration (yrs ) | 9.9 | 13.1 | 10.7 |
| Seropositivity (% ) | 78.7 | 75.9 | 69.8 |
| Previous b/tsDMARD (% ) | |||
| 0 | 34.0 | 30.8 | 59.7 |
| 1 | 20.9 | 25.9 | 24.3 |
| 2 | 16.6 | 21.7 | 10.4 |
| 3 or more | 28.5 | 21.5 | 5.6 |
| Concomitant GC (% ) | 44.6 | 50.7 | 41.3 |
| Concomitant CsDMARD (% ) | |||
| MTX | 22.6 | 22.0 | 28.8 |
| MTX + other | 9.5 | 9.7 | 13.1 |
| None | 50.5 | 52.5 | 43.5 |
| Other | 17.4 | 15.9 | 14.7 |
| CRP | 13.2 (24.1) | 13.3 (25.6) | 12.3 (24.1) |
| CDAI | 23.7 (13.8) | 22.9 (13.5) | 22.6 (14.0) |
| DAS 28 | 4.7 (1.5) | 4.7 (1.6) | 4.6 (1.6) |
| HAQ | 1.2 (0.7) | 1.2 (0.7) | 1.1 (0.7) |
| BMI | 27.1 (5.9) | 26.8 (5.8) | 26.8 (5.8) |
| Patients with at least one Comorbidity (% ) | 51.7 | 53.9 | 49.6 |
csDMARDs = classical synthetic DMARDs, MTX = methotrexate, GC = glucocorticoids, CRP = C-reactive protein, CDAI = Clinical Disease Activity Index, DAS 28 = Disease Activity Score 28, HAQ = Health Assessment Questionnaire, BMI = Body Mass Index, *Treatment duration (median [IQR]) = Last visit date – start date (if treatment is ongoing), treatment stop date – treatment start date (if treatment has stopped)
. 1 BARI (baricitinib; 44.41 %), FILGO (filgotinib; 0.23%), TOFA (tofacitinib; 49.59%), UPA (upadacitinib; 5.78%); 2 ABA (abatacept; 39.96%), ANAK (anakinra; 2.64%), SARI (sarilumab; 3.14%), TOCI (tocilizumab; 52.55%); 3 ADA (adalimumab; 31.00%), CERT (certolizumab; 8.33%), ETAN (etanercept; 38.83%), GOLI (golimumab; 9.36%), INFL (infliximab; 12.56%)
Results: 90,478 treatment courses were included in the analysis (
Comparison of cumulative incidence of treatment discontinuation for adverse events in JAKi, TNFi, and OMA group
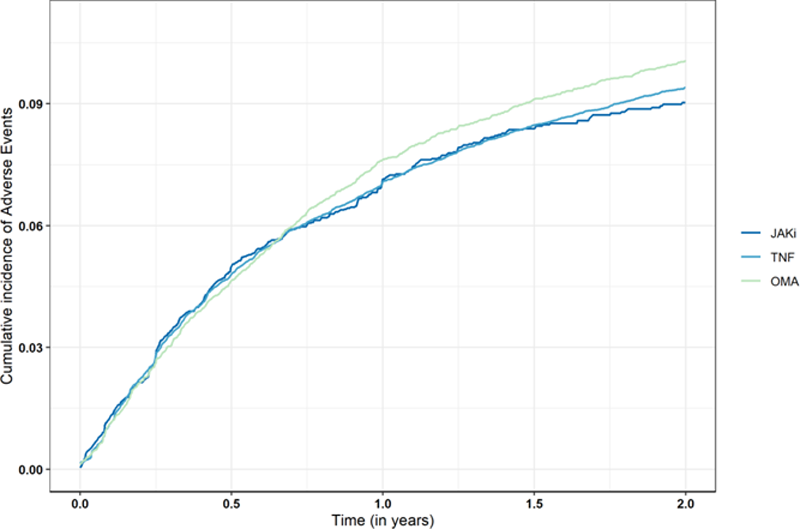
Conclusion: After adjusting for potential confounders, the rate of treatment discontinuation for AEs was comparable between JAKi and OMA or TNFi. Treatment discontinuation for AEs comprises a wide range of AEs; future analyses will be performed to investigate specific AEs, such as cancer, serious infections or major adverse cardiovascular events.
REFERENCES:
[1]Ann Rheum Dis 2022. doi: 10.1136/annrheumdis-2021-221915.
Disclosure of Interests: Eric Nham: None declared, Romain Aymon: None declared, Denis Mongin: None declared, Sytske Anne Bergstra: None declared, Denis Choquette Speakers bureau: DC reports speaker or consultant fees from Abbvie, Amgen, Eli Lilly, Fresenius-Kabi,Pfizer, Novartis, Sandoz, Tevapharm, Consultant of: Stated above, Catalin Codreanu Speakers bureau: CC reports speaker/consulting fees from AbbVie, Amgen, Astra Zeneca, Boehringer Ingelheim, Ewopharma, Lilly, Novartis, Pfizer, Richter, Consultant of: Stated above, Ori Elkayam Consultant of: OE has received consultant and honorary fees from Pfizer, Lilly, Abbvie, Novartis, Jansen, BI, Kimme Hyrich Speakers bureau: KLH has received speaker honoraria from Abbvie, Grant/research support from: KLH has received grant income from Pfizer and BMS, Florenzo Iannone Speakers bureau: FI has received consulting/speaker’s fees from Abbvie, BMS, Celgene, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, SOBI, Roche and UCB, Consultant of: Stated above, Nevsun Inanc Speakers bureau: NI has received consultant and speaker/honoraria from Abbvie, Lilly, MSD, Novartis, Pfizer, Roche, Amgen, Celltrion,UCB., Consultant of: Stated above, Lianne Kearsley-Fleet: None declared, Eirik kristianslund: None declared, Tore K. Kvien Speakers bureau: TKK has received fees for speaking and/or consulting from several companies among them Pfizer, AbbVie, Lilly and Galapagos/Gilead, Consultant of: Stated above, Burkhard Leeb Speakers bureau: BFL has received speaker honoraria from Sandoz, Abbvie, Eli-Lilly, Pfizer, Roche, Grünenthal, Biogen, Celgene, Galina Lukina Speakers bureau: GVL has received speaker fees from Abbvie, Lilly, Novartis, MSD, Roche, Pfizer, Dan Nordström Consultant of: DCN has acted as consultant for AbbVie, BMS, Lilly, MSD, Novartis, Pfizer, Roche and UCB, Karel Pavelka Speakers bureau: KP has received honoraria for lectures: MSD, Pfizer, Roche, Eli Lilly, Medac, UCB, SOBI, Biogen, Sandoz, Viatris, Manuel Pombo-Suarez Speakers bureau: MPS reports advisor and speaker honoraria from Janssen, Lilly, MSD, Novartis, Sanofi, Consultant of: Stated above, Ziga Rotar Speakers bureau: ZR has received fees for speaking/consulting from several companies among them Pfizer, AbbVie, and Eli Lilly, Consultant of: Stated above, Maria Jose Santos Speakers bureau: MJS has received speaker fees from Abbvie, AstraZeneca, Lilly, Novartis and Pfizer, Delphine Courvoisier: None declared, Kim Lauper Speakers bureau: KL reports speakers fees for Pfizer, Viatris and Celltrion, Consultant of: KL reports consulting fees for Pfizer, Axel Finckh Speakers bureau: AF reports honoraria and consultancies from Pfizer, BMS, MSD, Eli-Lilly, AbbVie, Galapagos, Mylan, UCB, Viatris, Consultant of: Stated above, Grant/research support from: AF reports grants from Pfizer INC, AbbVie, Galapagos, Eli Lilly