

Background: In axial spondyloarthritis (axSpA), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) is a key patient-reported outcome. However, one or more of its components may be missing when recorded in clinical practice.
Objectives: To determine whether an individual patient’s BASDAI at a given timepoint can be reliably calculated with different single imputation techniques and to explore the impact of the number of missing components and/or differences between missingness of individual components.
Methods: Real-life data from axSpA patients receiving tumour necrosis factor inhibitors (TNFi) from 13 countries in the European Spondyloarthritis (EuroSpA) Research Collaboration Network were utilized [1]. We studied missingness in BASDAI components based on simulations in a complete dataset, where we applied and expanded the approach of Ramiro et al. [2]. After introducing one or more missing components completely at random, BASDAI was calculated from the available components and with three different single imputation techniques: possible middle value (i.e. 50) of the component and mean and median of the available components. Differences between the observed (original) and calculated scores were assessed and correct classification of patients as having BASDAI<40 mm was additionally evaluated. For the setting with one missing component, differences arising between missing one of components 1-4 versus 5-6 were explored. Finally, the performance of imputations in relation to the values of the original score was investigated.
Results: A total of 19,894 axSpA patients with at least one complete BASDAI registration at any timepoint were included. 59,126 complete BASDAI registrations were utilized for the analyses with a mean BASDAI of 38.5 (standard deviation 25.9). Calculating BASDAI from the available components and imputing with mean or median showed similar levels of agreement (
Level of agreement between the original and calculated BASDAI and correct classification for BASDAI<40 mm
| Level of agreement with Dif≤6.9 mm* (%) | Correct classification for BASDAI<40 mm** (%) | ||
|---|---|---|---|
| 1 missing component | Available | 93.9 | 96.9 |
| Value 50 | 73.9 | 96.3 | |
| Mean | 94.2 | 96.8 | |
| Median | 93.1 | 96.8 | |
| 2 missing components | Available | 83.7 | 94.8 |
| Value 50 | 40.7 | 92.8 | |
| Mean | 83.5 | 94.8 | |
| Median | 82.8 | 94.7 | |
| 3 missing components | Available | 71.9 | 92.6 |
| Value 50 | 28.1 | 87.3 | |
| Mean | 72.2 | 92.6 | |
| Median | 69.7 | 92.2 | |
* The levels of agreement with a difference (Dif) of ≤6.9 mm between the original and calculated scores were based on the half of the smallest detectable change. Agreement of >90% was considered as acceptable. ** Correct classification of >95% was considered as acceptable.
Level of agreement between the original and calculated BASDAI and correct classification for BASDAI<40 mm as a function of the original score
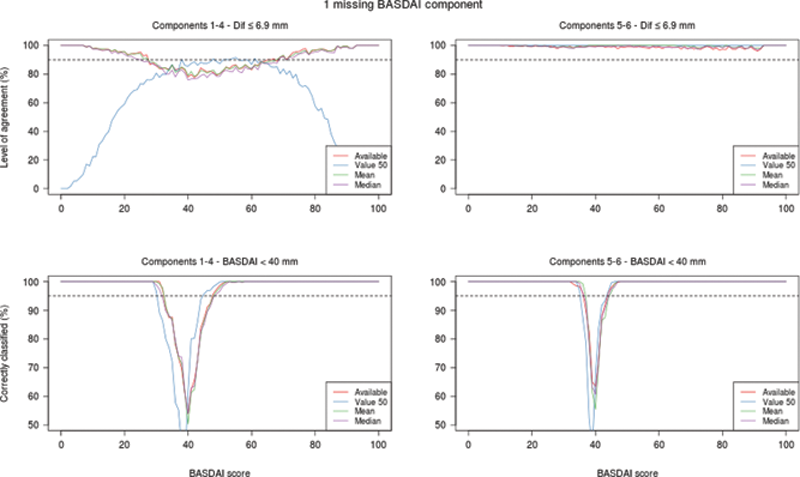
Conclusion: BASDAI calculation with available components gave similar results to single imputation of missing components with mean or median. Only when missing one of BASDAI components 5 or 6, single imputation techniques can reliably calculate individual BASDAI scores. However, missing any single component value results in misclassification of patients with original BASDAI scores close to 40.
REFERENCES:
[1]Ørnbjerg et al. (2019). Ann Rheum Dis , 78(11), 1536-1544.
[2]Ramiro et al. (2014). Rheumatology , 53(2), 374-376.
Acknowledgements: Novartis Pharma AG and IQVIA for supporting the EuroSpA collaboration.
Disclosure of Interests: Stylianos Georgiadis Grant/research support from: Novartis, Myriam Riek Grant/research support from: Novartis, Christos Polysopoulos Grant/research support from: Novartis, Almut Scherer Grant/research support from: Novartis, Daniela Di Giuseppe: None declared, Gareth T. Jones Speakers bureau: Janssen, Grant/research support from: AbbVie, Pfizer, UCB, Amgen, GSK, Merete Lund Hetland Grant/research support from: Abbvie, Biogen, BMS, Celltrion, Eli Lilly, Janssen Biologics B.V, Lundbeck Fonden, MSD, Medac, Pfizer, Roche, Samsung Biopies, Sandoz, Novartis, Mikkel Østergaard Speakers bureau: Abbvie, BMS, Boehringer-Ingelheim, Celgene, Eli-Lilly, Hospira, Janssen, Merck, Novartis, Novo, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi, UCB, Consultant of: Abbvie, BMS, Boehringer-Ingelheim, Celgene, Eli-Lilly, Hospira, Janssen, Merck, Novartis, Novo, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi, UCB, Grant/research support from: Abbvie, BMS, Merck, Celgene, Novartis, Simon Horskjær Rasmussen Grant/research support from: Novartis, Johan K Wallman Consultant of: AbbVie, Amgen, Celgene, Eli Lilly, Novartis, Bente Glintborg Grant/research support from: Pfizer, Abbvie, BMS, Anne Gitte Loft Speakers bureau: AbbVie, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, UCB, Consultant of: AbbVie, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, UCB, Karel Pavelka Speakers bureau: Pfizer, MSD, BMS, UCB, Amgen, Egis, Roche, AbbVie, Consultant of: Pfizer, MSD, BMS, UCB, Amgen, Egis, Roche, AbbVie, Jakub Zavada Speakers bureau: Abbvie, Elli-Lilly, Sandoz, Novartis, Egis, UCB, Consultant of: Abbvie, Elli-Lilly, Sandoz, Novartis, Egis, UCB, Merih Birlik: None declared, Ayten Yazici Grant/research support from: Roche, Brigitte Michelsen Grant/research support from: Novartis, Eirik kristianslund: None declared, Adrian Ciurea Speakers bureau: AbbVie, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Consultant of: AbbVie, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Michael J. Nissen Speakers bureau: AbbVie, Eli Lilly, Janssens, Novartis, Pfizer, Consultant of: AbbVie, Eli Lilly, Janssens, Novartis, Pfizer, Ana Maria Rodrigues Speakers bureau: Abbvie, Amgen, Consultant of: Abbvie, Amgen, Grant/research support from: Novartis, Pfizer, Amgen, Maria Jose Santos Speakers bureau: Abbvie, AstraZeneca, Lilly, Novartis, Pfizer, Gary Macfarlane Grant/research support from: GSK, Anna-Mari Hokkanen Grant/research support from: MSD, Heikki Relas Speakers bureau: Abbvie, Celgene, Pfizer, UCB, Viatris, Consultant of: Abbvie, Celgene, Pfizer, UCB, Viatris, Catalin Codreanu Speakers bureau: AbbVie, Amgen, Boehringer Ingelheim, Ewopharma, Lilly, Novartis, Pfizer, Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Ewopharma, Lilly, Novartis, Pfizer, Corina Mogosan: None declared, Ziga Rotar Speakers bureau: Abbvie, Novartis, MSD, Medis, Biogen, Eli Lilly, Pfizer, Sanofi, Lek, Janssen, Consultant of: Abbvie, Novartis, MSD, Medis, Biogen, Eli Lilly, Pfizer, Sanofi, Lek, Janssen, Matija Tomsic Speakers bureau: Abbvie, Amgen, Biogen, Eli Lilly, Janssen, Medis, MSD, Novartis, Pfizer, Sanofi, Sandoz-Lek, Consultant of: Abbvie, Amgen, Biogen, Eli Lilly, Janssen, Medis, MSD, Novartis, Pfizer, Sanofi, Sandoz-Lek, Björn Gudbjornsson Speakers bureau: Amgen, Novartis, Consultant of: Amgen, Novartis, Arni Jon Geirsson: None declared, Pasoon Hellamand Grant/research support from: Novartis, Marleen G.H. van de Sande Speakers bureau: Eli Lilly, Novartis, UCB, Janssen, Abbvie, Consultant of: Eli Lilly, Novartis, UCB, Janssen, Abbvie, Grant/research support from: Eli Lilly, Novartis, UCB, Janssen, Abbvie, Isabel Castrejon: None declared, Manuel Pombo-Suarez Consultant of: Abbvie, MSD, Roche, Bruno Frediani: None declared, Florenzo Iannone Speakers bureau: Abbvie, Amgen, AstraZeneca, BMS, Galapagos, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, UCB, Consultant of: Abbvie, Amgen, AstraZeneca, BMS, Galapagos, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, UCB, Lykke Midtbøll Ørnbjerg Grant/research support from: Novartis