

Background: Immunogenicity is a leading cause of treatment failure to TNF inhibitors, and also affects drug safety. Variations in HLA class II genes have been suggested to predispose to anti-drug antibody formation (ADA), but characterisation of biologically relevant HLA haplotypes, based on high-resolution genotyping, is lacking.
Objectives: To assess associations between HLA loci and formation of ADA to infliximab across different immune mediated inflammatory diseases.
Methods: Patients with immune mediated inflammatory diseases on infliximab therapy (N=612; 181 spondyloarthritis, 120 rheumatoid arthritis, 72 psoriatic arthritis, 114 ulcerative colitis, 80 Crohn’s disease and 45 psoriasis) participating in the Norwegian Drug Monitoring (NOR-DRUM) trials (1, 2) were included in the present analyses. Neutralising ADA were assessed with an automated fluorescence assay at each infusion. Next generation sequencing-based HLA typing was performed. Associations with ADA formation were assessed at locus, allele, haplotype and amino acid level. Peptide binding predictions for infliximab were performed.
Results: ADA were detected in 147 patients (24%). Significant associations were shown between ADA and several HLA loci, whereas conditional analyses indicated
HLA-DQB1
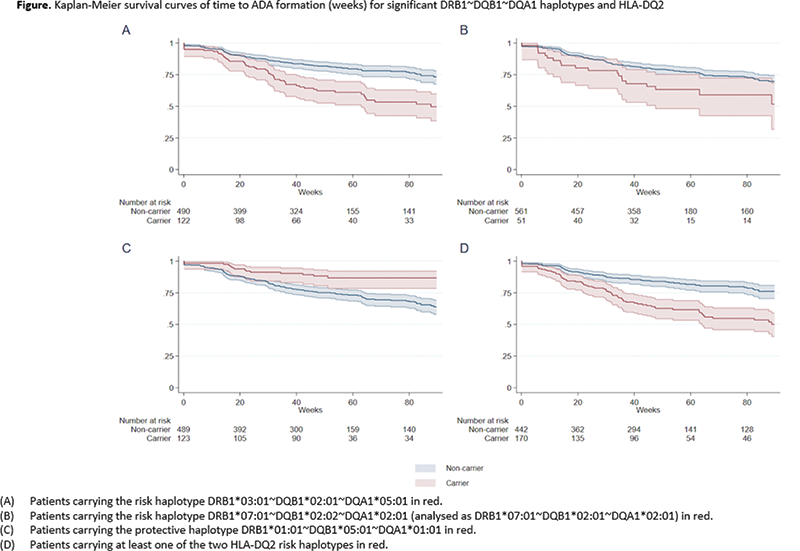
(p=1.4x10-6) as the primary risk locus. Highest risk of ADA formation was seen for patients carrying at least one of the HLA-DQ2 haplotypes; DQB1*02:01~DQA1*05:01 and DQB1*02:02~DQA1*02:01 (OR 3.18, 95% CI 2.15 to 4.69, p=5.9x10-9) (
HLA-DQ2 carrier frequencies according to the different disease phenotypes and for all diagnosis combined
| Diagnosis | HLA-DQ2 carrier-frequency among patients with ADA formation | HLA-DQ2 carrier-frequency among patients without ADA formation | P-value |
|---|---|---|---|
| RA (N=120) | 0.316 | 0.134 | 0.02 |
| PsA (N=72) | 0.55 | 0.231 | 0.01 |
| SpA (N=181) | 0.364 | 0.182 | 0.02 |
| UC (N=114) | 0.556 | 0.264 | 0.006 |
| CD (N=80) | 0.429 | 0.303 | 0.33 |
| Ps (N=45) | 0.867 | 0.267 | 0.0004 |
| All disease phenotypes | 0.469 | 0.217 | 5.9x10 -9 |
Conclusion: The risk of ADA to infliximab was three-fold higher in patients carrying the HLA-DQ2 risk haplotypes across diseases. A biological role for the HLA-DQ2 molecules encoded by the two different HLA-DQ2 risk haplotypes in the formation of ADA was further supported by peptide binding predictions. These novel findings provide promise for future incorporation of HLA-DQ2 testing to facilitate personalised treatment decisions.
REFERENCES:
[1]Syversen SW et al. Jama. 2021;326(23):2375-84.
[2]Syversen SW et al. Jama. 2021;325(17):1744-54.
Disclosure of Interests: Marthe Kirkesæther Brun: None declared, Kristin Hammersbøen Bjørlykke: None declared, Marte K. Viken: None declared, Bitte Stenvik Employee of: is a former employee of UCB Pharma, Rolf A. Klaasen: None declared, Johanna Gehin: None declared, David J Warren: None declared, Joe Sexton: None declared, Øystein Sandanger: None declared, Cato Mørk Speakers bureau: Novartis Norway, LEO Pharma, ACO Hud Norge, Cellgene, Abbvie, and Galderma Nordic AB., Consultant of: Novartis Norway, LEO Pharma, ACO Hud Norge, Cellgene, Abbvie, and Galderma Nordic AB., Tore K. Kvien Speakers bureau: Amgen, Celltrion, Evapharma, Gilead, Hikma, Mylan, Oktal, Pfizer, Sandoz, Sanofi, UCB, Consultant of: Amgen, Celltrion, Evapharma, Gilead, Hikma, Mylan, Oktal, Pfizer, Sandoz, Sanofi, UCB, Grant/research support from: AbbVie, Amgen, BMS, Novartis, Pfizer, UCB, Espen A Haavardsholm Speakers bureau: Pfizer, AbbVie, Celgene, Novartis, Janssen, Gilead, Eli-Lilly, and UCB, Consultant of: Pfizer, AbbVie, Celgene, Novartis, Janssen, Gilead, Eli-Lilly, and UCB, Jørgen Jahnsen Speakers bureau: AbbVie, Boerhinger Ingelheim, BMS, Celltrion, Giliad, Hikma, Janssen Cilag, Novartis, Orion Pharma, Pfizer, Roche, Takeda, and Sandoz, Consultant of: AbbVie, Boerhinger Ingelheim, BMS, Celltrion, Giliad, Hikma, Janssen Cilag, Novartis, Orion Pharma, Pfizer, Roche, Takeda, and Sandoz, Guro Løvik Goll Speakers bureau: Pfizer, AbbVie, Boehringer Ingelheim, Roche, Orion pharma, Sandoz, Novartis, and UCB, Consultant of: Pfizer, AbbVie, Boehringer Ingelheim, Roche, Orion pharma, Sandoz, Novartis, and UCB, Benedicte A. Lie: None declared, Kristin Kaasen Jørgensen Speakers bureau: Roche, BMS, Celltrion, and Norgine., Consultant of: Roche, BMS, Celltrion, and Norgine., Nils Bolstad Speakers bureau: Roche Pharmaceuticals and Novartis, Consultant of: Janssen, Silje Watterdal Syversen: None declared