

Background: The autoimmune regulator gene AIRE is the main controlling factor for developing central immunotolerance. Variants in AIRE lead to autoimmunity and underly the autoimmune polyendocrine syndrome type 1 (APS-1 or APECED), defined by the clinical triad chronic mucocutaneous candiasis hypoparathyroidism and adrenal insufficiency.(1, 2) Manifold additional manifestation have been described, including Sjögren-like disease lacking the typical serology.(3) Furthermore, serological abnormalities, e.g. antibodies against IL-22, IL-17F and IFNώ are a hallmark of the disease. Due to the diversity in clinical manifestations and the genetic variants in AIRE, diagnosis is often delayed.
Objectives: Case report of a patient with 2 new variants in AIRE with diverse rheumatological and autoimmune disorders as well as thymoma.
Methods: Case report.
Results: The 38-year-old male patient was transferred to our clinic for immunoglobuline therapy, from his treating neurologist. His medical history included diabetes mellitus type 1, diagnosed in 1996, followed by Grave’s disease in 2000, culminating in a thyroidectomy in 2017. In 2009 he underwent a thymectomy for a thymoma. He had been treated with a series of immunosuppressive therapies including prednisolone, rituximab and mycophenolate, as well as plasmapheresis and immunopheresis for his atypical, seronegative myasthenia gravis. Due to the limited effect of the previous therapeutic regimen a trial with intravenous immunoglobulin had been initiated inducing clinical stabilization.
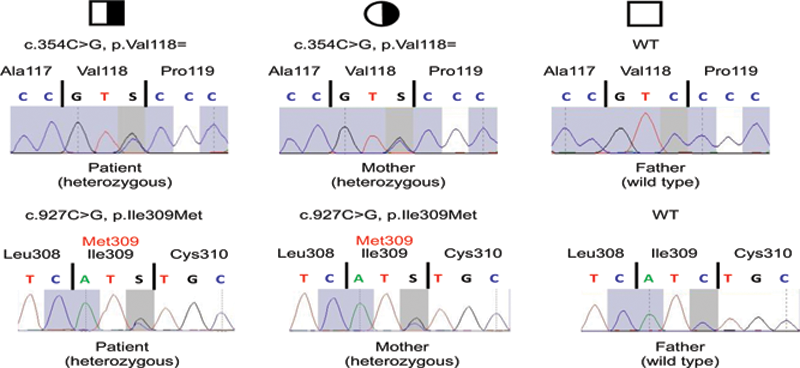
We diagnosed Sjogren-like syndrome based on sicca symptoms and a suspicious submandibular salivary gland in sonography, without typical serology. Family history was negative for autoimmune diseases. The clinical findings pointed towards immunodysregulation, prompting cytokine examination, lymphocyte phenotyping and genetic testing. Cytokines (IFNγ, IL-2, IL-4, IL-10, IL-17) under immunosuppression with 20 mg prednisolone and 2 g of mycophenolate were all low/below detection threshold. Flow cytometry showed no abnormalities except for a low CD20 (after Rituximab) and a slightly elevated T-reg count. Genetic testing revealed heterozygous variants in exon three (c.354C>G) and eight (c.927C>G) of
AIRE
. The patient´s mother showed the same genotype - indicating that the variants in the patient are
in cis
- but is asymptomatic. (
Electropherograms of the heterozygous patient, his heterozygous, asymptomatic mother and his asymptomatic father, who has a wildtype of AIRE.
Conclusion: To our knowledge this is the first report of a patient with thymoma and genetic variant(s) in the AIRE gene displaying several autoimmune diseases. Although clinical similarities between thymoma patients and APECED / APS1 are known (4), a genetic link between these 2 diseases has not yet been described. As rheumatic autoimmunity can be induced by either, rheumatologists should be aware of these potentially underlying causes.
An additional point of interest is the lack of autoimmunity in the mother with the same genetic variants, which suggests an additional environmental trigger (e.g. smoking, viral infection) in the patient.
REFERENCES:
[1]Aaltonen J, Björses P, Perheentupa J, Horelli–Kuitunen N, Palotie A, Peltonen L, et al. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nature Genetics. 1997;17(4):399-403.
[2]Ahonen P, Myllärniemi S, Sipilä I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322(26):1829-36.
[3]Ferre EM, Rose SR, Rosenzweig SD, Burbelo PD, Romito KR, Niemela JE, et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight. 2016;1(13).
[4]Wolff AS, Kärner J, Owe JF, Oftedal BE, Gilhus NE, Erichsen MM, et al. Clinical and serologic parallels to APS-I in patients with thymomas and autoantigen transcripts in their tumors. J Immunol. 2014;193(8):3880-90.
Disclosure of Interests: None declared