

Background: Infiltration of monocyte-derived macrophages into the synovial tissue (ST) is a hallmark of rheumatoid arthritis (RA) pathology. These macrophages promote inflammation, local joint effusion, and joint damage via the release of cytokines, oxygen reactive species, and tissue damaging enzymes. However, balancing these, are the ‘regulatory’ macrophages with inflammation-resolving properties, characterised by expression of CD206 and MerTK, dominant within the ST of healthy individuals as well as RA patients in remission (1). Indeed, these cells are believed to actively contribute to the maintenance of remission.
Macrophages are known to exhibit remarkable phenotypic plasticity and understanding the role of this characteristic in regulating inflammation and pathology remains a major challenge, as does the characterization of factors in the microenvironment such as the synovium that control such macrophage characteristics. Importantly, whether the infiltrating, inflammatory macrophages of the RA ST similarly exhibit such phenotypic plasticity, and whether this occurs during the process of reaching remission, remains to be studied.
Objectives: We investigated the phenotypic plasticity of inflammatory synovial macrophages from patients with RA in vitro, investigating their ability to convert from an inflammatory macrophage population into ‘regulatory’ CD206 + MerTK + macrophages. These findings will provide a proof-of-concept as to the utility of these macrophage for a cell-based therapy in resolving inflammation in patients with RA, and will likely extend our understanding of the mechanisms of action of currently used therapeutics.
Methods: Synovial fluid (SF) mononuclear cells were obtained from patients with active early RA (<1 year; fulfilling 2010 ACR/EULAR classification criteria). Cryopreserved SFMCs were cultured for 48hr in the presence of 10 ng/mL interferon(IFN)γ, 50 ng/mL dexamethasone, 10 μg/mL Infliximab, or diluent. Following culture, cells were immunostained and analysed using a Beckman Coulter CytoFLEX flow cytometer and FlowJo software. SF macrophages were characterised by expression of CD14, CD45, CD68 (
Synovial fluid CD68 + macrophage plasticity in vitro. (A) Gating strategy depicting CD68 + CD45 + CD14 + SF macrophage determination. (B) Proportions of CD206 and MerTK-expressing SF macrophages after 48hr culture in the presence of 10 ng/mL IFNγ, 50 ng/mL dexamethasone or 10 µg/mL Infliximab, or absence. Data are representative of 5 individual experiments. Data were analysed by two-way ANOVA followed by Dunnett’s multiple comparison test, *p<0.05.
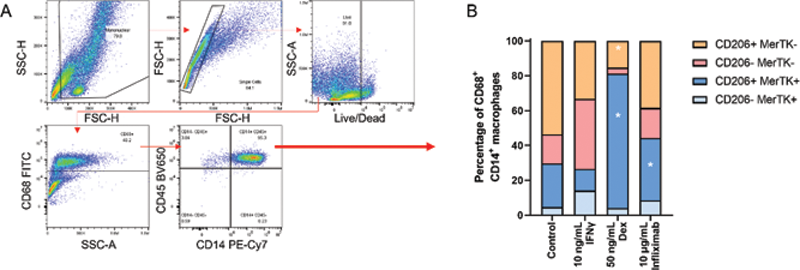
Results: Prior to culture, the CD68 + macrophage populations present in SF were found to be predominantly CD206 - MerTK - . After 48 hours of culture, in the absence of any stimulus, there was an increase in proportions of CD206 + MerTK + macrophages. Treatment with either dexamethasone or anti-TNF (Infliximab) resulted in a further increase in proportions of CD206 + MerTK + , M2-like macrophages. In contrast, culture with IFNγ induced a reduction in this population. Importantly, we found that the generated CD206 + MerTK + macrophages were phenotypically stable in culture following removal of these differentiating agents.
Conclusion: Our findings demonstrate that inflammatory SF cells are indeed able to polarise to regulatory, CD206 + MerTK + macrophages in vitro . The findings provide further mechanistic insights into the basis for the therapeutic benefits of glucocorticoids and TNF inhibitors, as well as providing initial proof-of-concept in the use of regulatory macrophages as a cellular-based therapy or therapeutic target for patients with RA.
REFERENCES:
[1]Alivernini S, MacDonald L, Elmesmari A, et al., Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nature Medicine. 2020;26(8):1295-306 10.1038/s41591-020-0939-8.
Disclosure of Interests: None declared