

Background: Treatment options for psoriasis (PsO) and psoriatic arthritis (PsA) have evolved significantly since the era of biologics. Clinical trials (mainly placebo-controlled for 12 to 16 weeks) are inadequate to assess the relative long-term efficacy of biologics and are often insufficient regarding safety.
Objectives: To assess the long-term persistence of different biologic classes to treat PsA and PsO.
Methods: This nationwide cohort study involved the administrative healthcare database of the French health insurance scheme linked to the hospital discharge database. We included, in two sub-cohorts, all adults with PsA and those with PsO, who were new users of biologics (not in the year before the index date) during 2015-2019. We excluded patients hospitalised for PsO in the PsA cohort and for PsA in the PsO cohort in the year before the index date. Persistence was defined as the time from biologic initiation to discontinuation and was estimated by the Kaplan-Meier method. Comparison of persistence by biologic class involved using propensity score-weighted Cox models (IPTW) and adjustment on specific systemic non-biologics (time-dependant variables).
Results: We included 6,531 patients with PsA (mean age 49±13 years, 45% male): 4,974 (76%) starting a TNFi, 803 (12%) an IL12/23i and 754 (12%) an IL17i. We included 16,892 patients with PsO (mean age 53±17 years, 50% male): 10,199 (60%) starting a TNF inhibitor (TNFi), 3,982 (24%) an IL12/23i, and 2,711 (16%) an IL17i. Overall 3-year persistence rates were 36% and 41% for PsA and PsO (
Kaplan Meier estimates of biologic treatment persistence in psoriasis and psoriatic arthritis cohorts TNFi: tumor necrosis factor inhibitor; ILi: interleukin inhibitor.
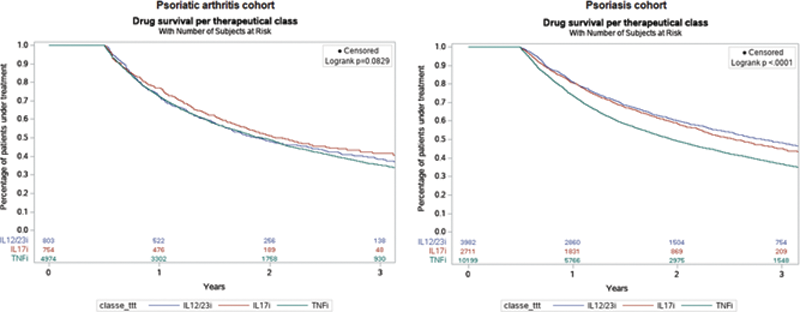
Conclusion: This real-life study suggests a higher persistence of IL17i than TNFi for PsA and PsO. IL17i also has better persistence than IL12/23i for PsA, with no difference for PsO. However, the persistence rates of all biologics remained globally low at 3 years.
Disclosure of Interests: Laura Pina Vegas: None declared, Laetitia Penso: None declared, Emilie Sbidian: None declared, Pascal Claudepierre Speakers bureau: P. Claudepierre has been an investigator for Roche Chugai, Sanofi Aventis, Celgene, Pfizer, MSD, Novartis and BMS., Consultant of: P. Claudepierre has received consulting fees from Abbvie, Amgen, Pfizer, Roche-Chugai, BMS, MSD, UCB, Novartis, Janssen, Lilly, Galapagos, Celgene (less than $10,000 each)