

Background: B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) are tumor necrosis factor (TNF) superfamily members that bind TACI (transmembrane activator and CAML interactor), BCMA (B-cell maturation antigen), and/or BAFF-R on B cells and together support B cell development, differentiation, and survival. ALPN-303 is an Fc fusion protein of a human TACI variant TNFR domain engineered by directed evolution 1,2 . It mediates significantly improved combined BAFF and APRIL inhibition in vitro and enhanced pharmacokinetic and immunomodulatory properties in preclinical studies, as compared to wild-type (WT) TACI-Fc molecules. B-cell targeting therapies like the WT TACI-Fc fusions atacicept and telitacicept have demonstrated promising clinical potential in B cell-related diseases like systemic lupus erythematosus (SLE). ALPN-303, with enhanced inhibitory activity against BAFF and APRIL, has previously been shown to demonstrate promising efficacy in an (NZB/NZW)F 1 mouse model of lupus, and may therefore further improve clinical outcomes in such diseases.
Objectives: To further characterize the comparative activity of ALPN-303 versus an Fc matched control, a WT TACI-Fc comparator (telitacicept), and/or a B cell-depleting therapy (anti-mouse CD20 [anti-mCD20] monoclonal antibody [mAb]), in antibody-related preclinical models.
Methods: The functional activity of ALPN-303, as compared to telitacicept or a depleting anti-mCD20 mAb, was evaluated in a sheep red blood cell (SRBC) immunization mouse model. Mice immunized intraperitoneally with SRBC on Study Day 0 were administered 200 µg ALPN-303 or a molar-matched amount (240 µg) of telitacicept on Days 1 and 6 or were treated with 200 µg anti-mCD20 (rat IgG2b) on Day 1. At study termination on Day 15, serum was collected to measure levels of test article and anti-SRBC immunoglobulin (Ig) titers, and spleens and bone marrow (BM) were collected for immunophenotyping by flow cytometry. A study in the inducible bm12 mouse model of lupus was also conducted, with mice treated twice weekly with 200 µg ALPN-303 or a molar-matched dose of Fc control, starting on Day 5 after splenocyte transfer and continuing through Week 13.
Results: ALPN-303 administration rapidly and potently reduced BM plasma cells, splenic germinal center B cells, follicular T helper cells, and plasmablasts in SRBC-immunized mice, often significantly more so than telitacicept and/or anti-mCD20 mAb. ALPN-303 also significantly reduced serum titers of anti-SRBC IgM, IgG1, IgG2a, and IgG2b as compared to all other treatment groups. In the bm12 model, ALPN-303 treatment significantly impacted the same key lymphocyte subsets affected in the SRBC model, and significantly reduced circulating anti-dsDNA antibodies (
ALPN-303 treatment significantly reduces serum autoantibody titers and renal immune complex deposition in the inducible bm12 mouse model of lupus. * p <0.05, ** p <0.01, **** p <0.0001 by the Kruskal-Wallis test.
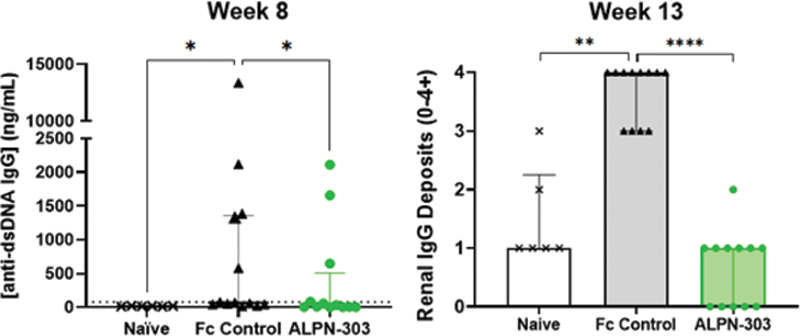
Conclusion: ALPN-303 is an engineered, potent BAFF/APRIL antagonist that continues to consistently demonstrate encouraging immunomodulatory activity and efficacy in vitro and in vivo, as further demonstrated in the SRBC immunization and bm12 lupus models, with superiority to WT TACI-Fc and anti-CD20 comparators. ALPN-303 may thus be an attractive development candidate for the treatment of multiple autoimmune and inflammatory diseases, particularly B cell- and/or autoantibody-related diseases such as SLE, Sjögren’s syndrome, and other connective tissue diseases. A Phase 1 study of ALPN-303 in adult healthy volunteers (NCT05034484) is ongoing.
REFERENCES:
[1]Dillon SR, Evans LS, Lewis KE, et al. Annals of the Rheumatic Diseases 2021;80:21.
[2]Dillon SR, Evans LS, Lewis KE, et al. Arthritis Rheumatol. 2021; 73 (suppl 10).
Disclosure of Interests: Stacey R. Dillon Employee of: Alpine Immune Sciences, Katherine E. Lewis Employee of: Alpine Immune Sciences Inc, Sherri Mudri Employee of: Alpine Immune Sciences Inc, Kayla Kleist Employee of: Alpine Immune Sciences Inc, Luana Griffin Employee of: Alpine Immune Sciences Inc, Lawrence S. Evans Employee of: Alpine Immune Sciences Inc, Janhavi Bhandari Shareholder of: Amgen, Novo Nordisk, Employee of: Alpine Immune Sciences Inc, Logan Garrett Employee of: Alpine Immune Sciences Inc, Michelle A. Seaberg Employee of: Alpine Immune Sciences Inc, NingXin Wang Employee of: Alpine Immune Sciences Inc, Allison Chunyk Employee of: Alpine Immune Sciences Inc, Daniel Ardourel Employee of: Alpine Immune Sciences Inc, LuAnne Hebb Employee of: Alpine Immune Sciences Inc, Martin F. Wolfson Employee of: Alpine Immune Sciences Inc, Mark W. Rixon Employee of: Alpine Immune Sciences Inc, Pamela Holland Employee of: Alpine Immune Sciences Inc, Stanford L. Peng Employee of: Alpine Immune Sciences Inc