

Background: CLIPPER2 was an 8-year, open-label extension of the phase 3b, multicenter, 2-year CLIPPER study of the safety and efficacy of etanercept (ETN) in the treatment of patients (pts) with juvenile idiopathic arthritis (JIA) categorized as extended oligoarticular arthritis (eoJIA), enthesitis-related arthritis (ERA), or psoriatic arthritis (PsA).
Objectives: The objective of this analysis was to describe the safety of ETN in this population after 10 years of follow up.
Methods: Pts (n=127) with eoJIA (2-17 years), ERA, or PsA (each 12-17 years) who received ≥1 ETN dose (0.8 mg/kg once weekly [max, 50 mg]) in CLIPPER were eligible to enter CLIPPER2. The primary outcome measure was the occurrence of malignancy. Long-term safety was assessed as the total incidence of events from CLIPPER baseline (BL) to month (mth) 120, frequency of events per 100 patient-years (EP100PY), and frequency of events in each study year.
Results: A total of 109/127 (86%) pts entered CLIPPER2; 99 (78%) continued in the active treatment period. At mth 120, 84 (66%) pts had completed the study; 27 (21%) while actively taking ETN; 7 (6%) had withdrawn from treatment due to low/inactive disease; 5 (4%) had re-started ETN following an earlier withdrawal from treatment; and 45 (35%) had stopped ETN (but remained under observation); 25 (20%) pts permanently discontinued from the CLIPPER2 study. In CLIPPER/CLIPPER2, 1 case of malignancy (Hodgkin’s disease) was reported (1 pt with eoJIA in Year 3). There was 1 case of uveitis (1 pt with eoJIA in Year 8) and 3 of Crohn’s disease (2 pts with ERA, Year 1/Year 6; 1 pt with eoJIA, Year 5). There were 2 cases of opportunistic infections (both herpes zoster), and no deaths. Overall, there were 559 (81.82 EP100PY) treatment-emergent adverse events (TEAEs) excluding infections and injection-site reactions (ISRs). The overall rate of TE serious infections was low (N=14; 2.05 EP100PY) (
ETN Safety Summary (from CLIPPER BL to mth 120), N (EP100PY) (FAS)*
| eoJIA, n=60(EXP=313.667 PY) | ERA, n=38(EXP=206.971 PY) | PsA, n=29(EXP=162.576 PY) | Total, n=12(EXP=683.214 PY) | |
|---|---|---|---|---|
| TEAEs† | 269 (85.76) | 176 (85.04) | 114 (70.12) | 559 (81.82) |
| TE serious AEs† | 16 (5.10) | 17 (8.21) | 7 (4.31) | 40 (5.85) |
| TE ISRs | 23 (7.33) | 29 (14.01) | 12 (7.38) | 64 (9.37) |
| TE infections | 418 (133.26) | 99 (47.83) | 155 (95.34) | 672 (98.36) |
| TE serious infectionsǂ | 5 (1.59) | 4 (1.93) | 5 (3.08) | 14 (2.05) |
| Opportunistic infections§ | 0 | 1 (0.48) | 1 (0.62) | 2 (0.29) |
| TEAEs causing withdrawal† | 7 (2.23) | 9 (4.35) | 2 (1.23) | 18 (2.63) |
| TE infections causing withdrawal | 2 (0.64) | 0 | 1 (0.62) | 3 (0.44) |
*While on active ETN treatment or within 30 days of last dose
†Excluding infections/ISRs
ǂGastroenteritis, 2 (0.29); acute tonsillitis, anal abscess, bronchopneumonia, gastrointestinal infection, helicobacter gastritis, influenza, peritonitis, pharyngitis, pyelocystitis, sepsis, urinary tract infection, viral infection, all 1 (0.15)
§Both herpes zoster
EXP, exposure to ETN; FAS, full analysis set; n, number of patients; N, number of events
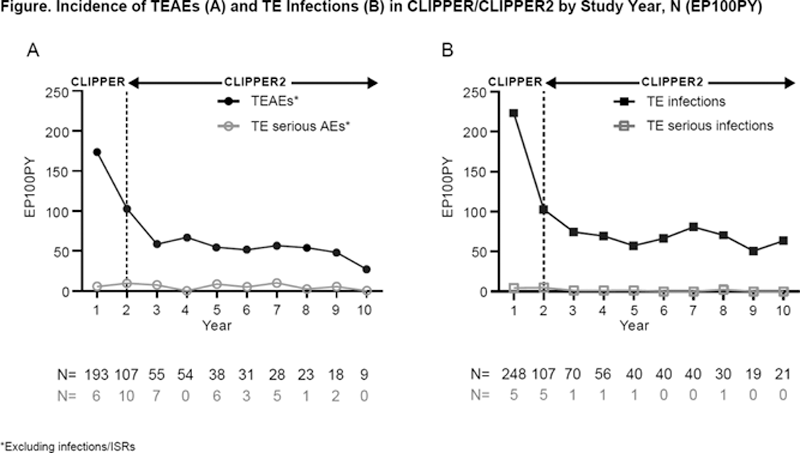
Conclusion: ETN treatment to mth 120 was well tolerated in this patient population and consistent with the known safety profile. Frequency of TEAEs and TE infections decreased over time. Over 10 years, there was 1 reported event of malignancy and the overall rate of TE serious infections was low.
REFERENCES:
[1]NCT00962741/NCT01421069
Acknowledgements: Medical writing support was provided by Iain McDonald, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Disclosure of Interests: Jelena Vojinovic Speakers bureau: Abbvie, Roche, Sandoz, Joke Dehoorne Speakers bureau: Abbvie, Roche, Consultant of: Abbvie, Roche, Violeta Panaviene: None declared, Gordana Susic: None declared, Gerd Horneff Speakers bureau: Chugai, Eli-Lilly, Glaxo Smith and Kline, Janssen, Novartis, Pfizer, Roche and Sobi, Grant/research support from: Novartis, Janssen, Roche, Valda Stanevicha Speakers bureau: Sandoz, Abbvie, Roche, Katarzyna Kobusinska: None declared, Zbigniew Żuber: None declared, Bogna Dobrzyniecka: None declared, Jonathan Akikusa: None declared, Tadej Avcin Speakers bureau: AbbVie, Octapharma, and Takeda, Consultant of: AbbVie, Alexion, Octapharma, and Takeda, Alberto Martini Speakers bureau: Aurinia, Bristol Myers and Squibb, Eli-Lilly, EMD Serono, Janssen, Pfizer, Roche, Consultant of: Aurinia, Bristol Myers and Squibb, Eli-Lilly, EMD Serono, Janssen, Pfizer, Roche, Cecilia Borlenghi Shareholder of: Pfizer, Employee of: Pfizer, Edmund Arthur Employee of: Pfizer, Svitlana Y Tatulych Shareholder of: Pfizer, Employee of: Pfizer, Chuanbo Zang Shareholder of: Pfizer, Employee of: Pfizer, Bonnie Vlahos Shareholder of: Pfizer, Employee of: Pfizer, Nicolino Ruperto Speakers bureau: Ablynx, Amgen, Astrazeneca-Medimmune, Aurinia, Bayer, Bristol Myers and Squibb, Cambridge Healthcare Research (CHR), Celgene, Domain therapeutic, Eli-Lilly, EMD Serono, Glaxo Smith and Kline, Idorsia, Janssen, Novartis, Pfizer, Sobi, UCB., Consultant of: Ablynx, Amgen, Astrazeneca-Medimmune, Aurinia, Bayer, Bristol Myers and Squibb, Cambridge Healthcare Research (CHR), Celgene, Domain therapeutic, Eli-Lilly, EMD Serono, Glaxo Smith and Kline, Idorsia, Janssen, Novartis, Pfizer, Sobi, UCB.