

Background: SLE is a heterogeneous disease with diverse clinical presentations, and up to 70–80% of patients develop skin manifestations. 1–3 In SLE, plasmacytoid dendritic cells (pDCs), a major source of Type I interferon (IFN), accumulate in the skin. 4 Treatment with BIIB059, a humanized monoclonal antibody targeting blood dendritic cell antigen 2 (BDCA2) that is expressed on pDCs, leads to rapid internalization of BDCA2 from the surfaces of pDCs and inhibits the production of Type I IFNs, pro-inflammatory cytokines, and chemokines. 5 Part A of the randomized, two-part, Phase 2 LILAC study (NCT02847598) enrolled participants with SLE and active skin and joint disease. The primary endpoint was met, with a greater reduction in total active joint count at Week 24 in the BIIB059 treatment group vs placebo (PBO), and more participants achieved a ≥50% improvement from baseline in Cutaneous Lupus Erythematosus Disease Area and Severity Index – Activity (CLASI-A) score with BIIB059 vs PBO. 6
Objectives: To further evaluate the effect of BIIB059 vs PBO in reducing skin disease activity, as measured by various CLASI-A response thresholds.
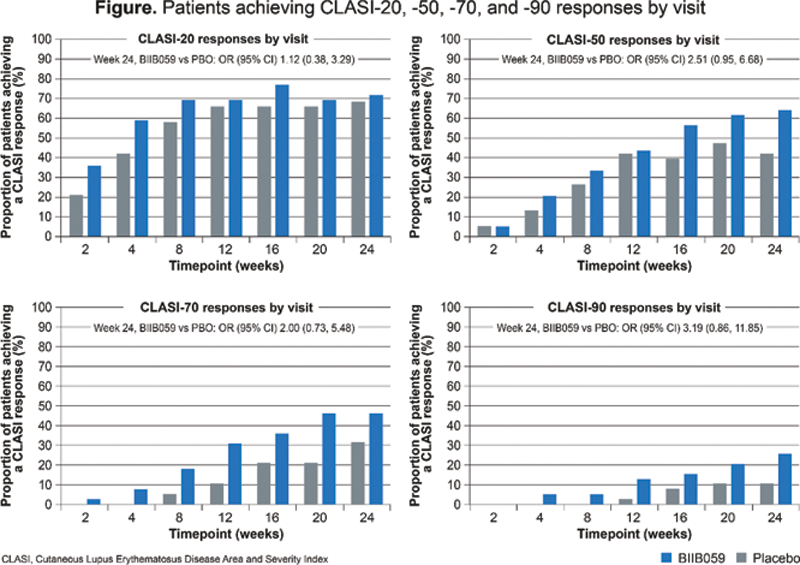
Methods: Adults with an SLE diagnosis according to the revised ACR 1997 SLE classification criteria, with ≥4 tender and ≥4 swollen joints (28-joint assessment), active skin disease (as defined by the SLE Disease Activity Index 2000 [SLEDAI-2K]), and positive anti-nuclear antibodies and/or anti-double-stranded DNA antibodies, were enrolled. Participants were randomized to receive BIIB059 450 mg or PBO, administered subcutaneously every 4 weeks with an additional dose at Week 2. Improvements in skin disease were assessed in participants with baseline CLASI-A score ≥8. The proportion of participants achieving a ≥7-point reduction from baseline in CLASI-A score was assessed at Week 24, and CLASI-20, -50, -70, and -90 responses were assessed over time. Achievement of CLASI-A scores of 0–1 was also assessed at Week 24. These analyses used non-responder imputation with logistic regression, without correction for multiplicity. The proportions of participants achieving CLASI-A scores of 0–3 and with resolution of SLEDAI-2K skin rash at Week 24 were evaluated ad hoc in the same population. Non-responder imputation was applied to visits post treatment failure and treatment discontinuation. Improvement from baseline in British Isles Lupus Assessment Group index (BILAG-2004) A or B mucocutaneous domains was similarly assessed at Week 24. P-values were calculated based on the odds ratios (ORs) for BIIB059 compared with PBO.
Results: At Week 24, a significantly greater proportion of participants receiving BIIB059 (n=39) vs PBO (n=38) had a ≥7-point reduction in CLASI-A score from baseline to Week 24 (56.4% vs 34.2%, OR [95% confidence interval {CI}] 2.71 [1.03, 7.17], P=0.044). Numerically greater proportions of participants receiving BIIB059 vs PBO achieved CLASI-50, CLASI-70, or CLASI-90 responses (
Conclusion: Numerically greater reductions in skin disease activity were consistently observed with BIIB059 treatment vs PBO in participants with SLE and active skin disease, supporting a potential benefit of BIIB059 treatment for skin manifestations in SLE.
REFERENCES:
[1]Dörner T, Furie R. Lancet 2019;393:2344–2358
[2]Patel J, et al. Curr Rheumatol Rep 2020;22:69
[3]Grönhagen C, et al. Lupus 2010;19:1187–1194
[4]Vermi W, et al. Immunobiology 2009;214:877–886
[5]Pellerin A, et al. EMBO Mol Med 2015;7:464–476
[6]Furie R, et al. Arthritis Rheumatol 2020;72(Suppl. 10):0935 (Abstract)
Acknowledgements: The authors thank the LILAC investigators for their valuable contributions to this study. This study was sponsored by Biogen (Cambridge, MA, USA). Writing and editorial support was provided by Selene Medical Communications (Macclesfield, UK), funded by Biogen.
Disclosure of Interests: Ronald van Vollenhoven Speakers bureau: AbbVie, Galapagos, GSK, Janssen, Pfizer, R-Pharma, UCB, Consultant of: AbbVie, AstraZeneca, Biogen, BMS, Galapagos, Janssen, Miltenyi, Pfizer, UCB, Grant/research support from: BMS, GSK, UCB (research support; institutional grants); MSD, Pfizer, Roche (educational program support; institutional grants), Richard Furie Consultant of: AstraZeneca, Biogen, Grant/research support from: AstraZeneca, Biogen, Victoria Werth Consultant of: Biogen, Grant/research support from: Biogen, Kenneth Kalunian Consultant of: AbbVie, Amgen, AstraZeneca, Aurinia, Biogen, Bristol Myers Squibb, Eli Lilly, Equillium, Genentech, Gilead, ILTOO, Janssen, Nektar, Roche, Viela, Grant/research support from: Lupus Research Alliance, Pfizer, Sanford Consortium, XIAOBI HUANG Shareholder of: Biogen, Employee of: Biogen, Cristina Musselli Shareholder of: Biogen, Employee of: Biogen, Catherine Barbey Shareholder of: Biogen, Employee of: Biogen, NATHALIE FRANCHIMONT Shareholder of: Biogen, OMass Therapeutics, Consultant of: OMass Therapeutics, Employee of: Biogen