

Background: Atacicept is a fusion protein that blocks B-lymphocyte stimulator and a proliferation-inducing ligand, which are increased in patients with SLE. APRIL-SLE was a double-blind, placebo-controlled, Phase 2 study that randomized patients with moderate-to-severe systemic lupus erythematosus (SLE) to atacicept 75 mg, atacicept 150 mg, or placebo twice-weekly for 4 weeks, then weekly for 48 weeks.
Objectives: The primary results of the APRIL-SLE study – the effect of atacicept compared to placebo in preventing new flares in patients with moderate-to-severe SLE – have been reported (Isenberg et al., 2013). We performed a post hoc analysis to describe the effect of atacicept compared to placebo on measures of renal function in patients with SLE; this effect has not been reported previously.
Methods: The APRIL-SLE study excluded patients with moderate to severe glomerulonephritis, as defined by either of the following: urinary protein/creatinine ratio (UPCR)>1 mg/mg and/or hematuria or a significant renal impairment as defined by estimated glomerular filtration rate (eGFR)<50 mL/min/1.73 m2. Patients with proteinuria and mild to moderate chronic kidney disease, as assessed by KDIGO criteria were eligible. UPCR and eGFR were measured at baseline, week 2, and then every 4 weeks until week 52. The median change from baseline to each of these timepoints was calculated for eGFR and UPCR using the Safety Analysis Set, comprised of all randomized patients who received at least 1 dose of study medication. Enrollment in the atacicept 150 mg group was discontinued prematurely due to 2 deaths from pneumonias. When treatment was discontinued, 62 of 144 patients in this group had completed 52 weeks of treatment; 27 other patients had already been withdrawn for various reasons; and, in the remaining 55 patients, treatment was stopped early as a safety precaution. Patients in the other two groups completed the protocol.
Results: In total, 111 patients in the placebo group, 112 patients in the atacicept 75 mg group, and 62 patients in the atacicept 150 mg group completed 52 weeks of treatment. The eGFR time course was stable for the atacicept groups compared to a 4.4% decline in the placebo group from baseline at week 52 (
Median Percent Change from Baseline of Estimated Glomerular Filtration Rate (eGFR) and Proteinuria at Week 52 – Safety Analysis Set
| Variable | Placebo | Atacicept 75 mg | Atacicept 150 mg |
|---|---|---|---|
| eGFR (mL/min) | n=110 | n=111 | n=62
|
| median | -4.35 | -1.49 | 0.57 |
| UPCR (mg/mg) | n=108 | n=108 | n=63 |
| median | 6.29 | -6.27 | -12.72 |
| UPCR (mg/mg)
| n=12 | n=15 | n=8 |
| median | 26.11 | -54.42 | -12.15 |
eGFR=estimated glomerular filtration rate; UPCR=urinary protein/creatinine ratio.
a Among patients with screening UPCR ≥0.2 mg/mg.
b Enrollment in the atacicept 150 mg arm was discontinued prematurely (described in Isenberg et al., 2015).
Median Change from Baseline in eGFR.
eGFR= estimated glomerular filtration rate; IQR=interquartile range
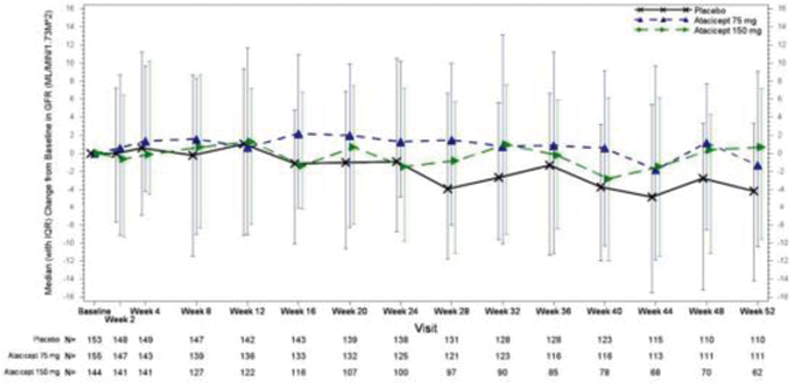
Conclusion: Results from this double-blind, placebo-controlled, Phase 2 study suggest a potential for improved renal function with atacicept treatment of patients with moderate-to-severe SLE.
REFERENCES:
[1]Isenberg D, Gordon C, Licu D, Copt S, Rossi CP, Wofsy D. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann Rheum Dis. 2015;74(11):2006-15. Erratum in: Ann Rheum Dis. 2016 May;75(5):946.
Disclosure of Interests: David Isenberg Consultant of: Professor Isenberg has consulted for Veratx, Servier, Astro-Zeneca, Idorsia, Merck Serono, and Amgen. His honoraria are passed onto a local arthritis charity., Celia J. F. Lin Shareholder of: Dr. Lin is an employee of Vera Therapeutics, Inc., Employee of: Dr. Lin is an employee of Vera Therapeutics, Inc., Amy Kao Shareholder of: Dr. Kao own stocks of Merck KGaA, Darmstadt, Germany, Employee of: Dr. Kao is an employee of EMD Serono Research & Development Institute, Inc (a business of Merck KGaA), Aida Arselan Aydemir Employee of: Ms. Aydemir is an employee of EMD Serono Research & Development Institute, Inc (a business of Merck KGaA), Caroline Gordon Speakers bureau: Dr. Gordon reports personal fees for speakers bureau from UCB, Consultant of: Dr. Gordon reports personal fees for honoraria from consultancy work from the Center for Disease Control and Prevention, Amgen, Astra-Zeneca, AbbVie, EMD Serono, MGP, Sanofi, and UCB, Grant/research support from: Dr. Gordon reports an educational grant from UCB to Sandwell and West Birmingham Hospitals NHS Trust that supported previous research work unrelated to any specific drug (last payment July 2019).