

Background: Published data suggest no increased rate of flare of autoimmune inflammatory rheumatic diseases (AIIRD) after COVID-19 mRNA vaccination; however, the studies are limited by small sample size, short follow up or at risk of selection bias (voluntary physician reports or patient surveys).
Objectives: To study flares of AIIRD within three months of the first dose of an anti-SARS-COV2 mRNA vaccine.
Methods: A retrospective cohort study of consecutive AIIRD patients ≥ 12 years old, across six public hospitals in Singapore who received at least one dose of an mRNA (Pfizer/BioNTech or Moderna) vaccine. Data were censored at the first post-vaccine clinic visit when the patient had flared or if ≥ three months had elapsed since the first dose of the vaccine, whichever came first. Predictors of flare were determined by Cox proportional hazards analysis and time to flare was examined using a Nelson Aalen cumulative hazard estimate (
Nelson-Aalen curve of flares over time
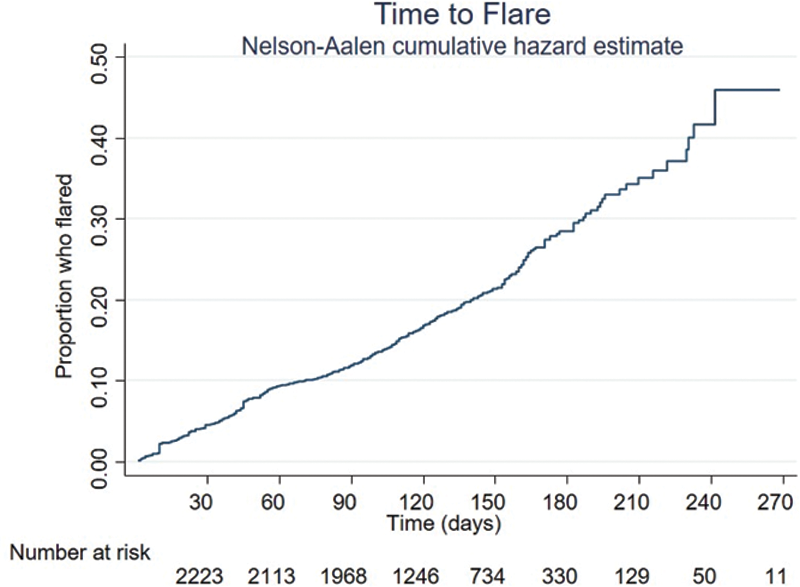
Results: 2339 patients (74% Chinese, 72% female) of median (IQR) age 64 (53, 71) years were included in the interim analysis (
Patient characteristics
| Baseline characteristics | No flares(n = 1887, %) | Flares within 0—3 months of 1 st vaccine dose (n= 272, %) | Flares outside of 0 – 3 months after 1 st vaccine dose (n = 180, %) |
|---|---|---|---|
| Age (median years, IQR) | 64 (53, 71) | 61 (50, 69) | 65 (55, 71) |
| Race | |||
| Chinese | 1386 (73) | 206 (76) | 129 (72) |
| Malay | 193 (10) | 28 (10) | 20 (11) |
| Indian | 195 (10) | 27 (10) | 26 (14) |
| GenderFemale | 1367 (72) | 200 (74) | 117 (65) |
| Vaccine type | |||
| Pfizer/BioNTech | 1713 (92) | 239 (90) | 160 (90) |
| Moderna | 149 (8) | 28 (10) | 18 (10) |
| Diagnosis | |||
| Rheumatoid Arthritis | 831 (44) | 139 (51) | 93 (52) |
| Systemic Lupus Erythematosus | 269 (14) | 20 (7) | 9 (5) |
| Psoriatic Arthritis | 225 (12) | 42 (15) | 29 (16) |
| Spondyloarthropathies | 141 (7) | 21 (7) | 17 (9) |
| Sjogren’s Syndrome | 114 (6) | 15 (6) | 8 (4) |
| Systemic sclerosis | 94 (5) | 4 (1) | 6 (3) |
| Baseline Physician Disease Activity | |||
| Remission | 1007 (53) | 99 (36) | 63 (35) |
| Low Disease Activity | 731 (39) | 128 (47) | 97 (54) |
| Moderate Disease Activity | 134 (7) | 40 (15) | 20 (11) |
| High Disease Activity | 15 (1) | 5 (2) | 0 |
452 (19%) flares were recorded during 9798.8 patient-months [4.6/100 patient-months, median (IQR) follow up duration 4.2 (3.3, 5.3) months], of which 272 (11.6%) patients flared within the 3-month period of interest and 180 (7.7%) flared outside of the 3-month period (
On multivariate Cox regression analysis, patients in the oldest age tertile [median (IQR) 74 (71, 79) years] were less likely to flare [HR 0.80 (95% CI 0.63, 1.00), p = 0.05] Patients with inflammatory arthritis (compared with connective tissue disease, vasculitis and others) and patients with baseline active disease were more likely to flare [HR 1.72 (95% CI 1.35, 2.20), p < 0.001 and 1.82 (95% CI 1.39, 2.39), p < 0.001 respectively]
Conclusion: There was a moderately high rate of AIIRD flares after mRNA vaccination; however, there was no clustering of flares in the immediate post-vaccine period to suggest causality. Older patients were less likely to flare, while those with inflammatory arthritis and active disease at baseline were more likely to flare.
Disclosure of Interests: Margaret Ma Grant/research support from: Support grant from multiple companies for the Singapore Biologics registry, Amelia Santosa Speakers bureau: Amgen Talk, Consultant of: Pfizer ad board, Kok Ooi Kong: None declared, Chuanhui Xu: None declared, Johnston Tang Gin Xiang: None declared, Gim Gee Teng Speakers bureau: Boehringer Ingleheim, Anselm Mak Speakers bureau: J&J and GSK, Grant/research support from: GSK - the supported studies programme, Sen Hee Tay: None declared, Victoria Wei Wen Ng: None declared, Joshua Zhi En Koh: None declared, Warren Fong Speakers bureau: speaker for Abbvie, DKSH, GSK, Novartis, Li-Ching Chew Speakers bureau: pfizer and Abbvie, Consultant of: Pfizer and Abbvie Advisory Board meeting, Grant/research support from: Abbvie educational grant for ultrasound conference, Andrea Low Speakers bureau: Boehringer Ingeilheim, Consultant of: Consultant/steering group committee for BI and J&J, annie law: None declared, Yih Jia Poh: None declared, Siaw Ing Yeo Grant/research support from: Multiple pharmaceutical companies for the support of the National Biologics Registry, Ying Ying Leung Speakers bureau: Abbvie, DKSH, Jassen, Novartis and Pfizer, Wei-Rui Goh: None declared, Chuah Tyng Yu: None declared, Nur Emillia Roslan: None declared, Stanley Angkodjojo Speakers bureau: Boehringer Ingeilheim, Consultant of: Abbvie and DKSH, Kee Fong Phang: None declared, Thaschawee Arkachaisri: None declared, Melonie Sriranganathan: None declared, Teck Choon TAN: None declared, Peter Cheung Consultant of: Ad board for Boehringer Ingleheim, novartis, janssen and abbvie, Grant/research support from: Novartis, Manjari Lahiri Speakers bureau: J&J, DSKH, Consultant of: DSKH, Gilead, Grant/research support from: Multiple pharma companies contributed to the Singapore Biologics registry
Novartis