

Background: Vagus nerve stimulation (VNS) activates innate neuroimmune reflexes that have been shown to reduce pro-inflammatory cytokines and clinical disease activity in subjects with rheumatoid arthritis (RA) .1 We previously reported primary clinical outcomes from a first-in-human, double-blind study of a novel, implanted VNS device called a MicroRegulator (MR). That study showed 5/10 subjects with drug-refractory RA met or exceeded the minimal clinically important difference (MCID) in DAS28-CRP; 2 subjects achieved DAS28 remission (DAS28-CRP < 2.6); and pro-inflammatory cytokines were decreased by >30% following 12 weeks of VNS. 2 We now report 24-week efficacy and safety findings from this study.
Objectives: Determine the long-term safety and exploratory efficacy of neurostimulation using a novel, miniaturized VNS device in patients with multiple drug refractory RA who were previously enrolled in a first in human study.
Methods: The primary study enrolled adult patients with active moderate-to-severe RA with incomplete response or intolerability to at least two biologic and/or targeted synthetic DMARDs having at least two different mechanisms of action. The study was enrolled in 2 Stages: Stage 1 (n=3) was open-label, and Stage 2 (n=11) was randomized and sham-controlled (
Study Schematic
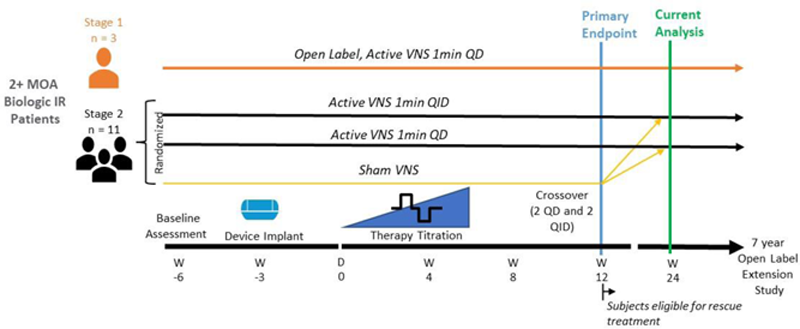
Results: There were no device-related adverse events from Week 12 through Week 24. Improvement in clinical disease activity was sustained through Week 24: 5/9 patients within the original, active VNS treatment groups met or exceeded EULAR response criteria for DAS28-CRP at Week 24 (
Change in DAS28-CRP at Week 24
| Subject | Treatment | DAS28-CRP (MCID -1.2) *added b/tsDMARD co-therapy |
|---|---|---|
| 005-01 | QD | -2.4 |
| 005-03 | QD | -2.21 |
| 006-01 | QD | -0.07 |
| 002-01 | QD | -4.95 * |
| 005-06 | QD | -0.72 * |
| 006-03 | QID | -0.69 |
| 008-01 | QID | 0.49 |
| 008-03 | QID | -3.14 |
| 008-04 | QID | 1.43 |
| 005-05 | Sham to QD | 0.39 |
| 006-04 | Sham to QD | -0.78 |
| 006-02 | Sham to QID | -0.22 |
| 008-02 | Sham to QID | -0.09 |
Conclusion: Improvements in clinical disease activity and pro-inflammatory cytokine suppression were maintained through 24 weeks of VNS treatment. Safety outcomes continue to support the risk/benefit profile of VNS as a treatment option for patients with multiple-drug refractory RA.
REFERENCES:
[1]Koopman PNAS 2016
[2]Genovese et al. Lancet Rheum 2020.
Acknowledgements: Authors wish to thank the patients for participating in the study
Disclosure of Interests: Norman Gaylis Grant/research support from: Primary investigator at AARDS Research Inc, Mark C. Genovese Shareholder of: Gilead Sciences, Consultant of: SetPoint Medical Inc, Vorso, InMedix, Galvani, Employee of: Gilead Sciences, David Sikes: None declared, Alan Kivitz Shareholder of: Amgen, Gilead, GSK, Sanofi, Pfizer, Speakers bureau: Abbvie, Celegene, Pfizer, Horizon, Merck, Genzyme, Sanofi, Flexion, Paid instructor for: Abbvie, Celegene, Pfizer, Horizon, Merck, Genzyme, Sanofi, Flexion, Consultant of: Abbvie, Janssen, Pfizer, Genzyme, Sanofi, Regeneron, Sun Pharma, Boehringer Ingelheim, Gilead, Diane M Horowitz: None declared, Charles Peterfy Speakers bureau: Amgen, Bristol-Myers Squibb, Consultant of: Abbvie, Five Prime, Genentech, Modern Bioscience, Myriad, Novartis, Roche, SetPoint Medical, Vorso, Employee of: Spire Sciences Inc, Yaakov Levine Employee of: Setpoint Medical, Melissa Evangelista Employee of: SetPoint Medical, David Chernoff Employee of: SetPoint Medical