

Background: Glucocorticoids (GC) are the cornerstone of treatment in Polymyalgia rheumatica (PMR) [1]. However, they are associated with considerable toxicity and inefficacy in part of the patients. Rituximab (RTX) was effective for PMR in a 21-week randomized controlled trial (RCT), however results from longer follow-up is still absent [2].
Objectives: To assess, in a randomized double blinded fashion, the clinical and GC-sparing effects one year after RTX.
Methods: In the BRIDGE-PMR, an RCT of 38 recently diagnosed and 9 relapsing PMR (2012 EULAR/ACR classification criteria) patients recruited from the Sint Maartenskliniek, patients were randomly allocated in a 1:1 ratio and treated with 1x 1000mg RTX / placebo (PCB) iv, identical pre-medication and an accelerated GC tapering protocol. After the 21-week study, patients were assessed in a double blinded prospective extension study up to one year after infusion. The primary outcome at one year was between group difference in GC-free remission (PMR-activity score < 10). Analysis was performed with Fischer’s exact test and a two-tailed p-value < 0.05 was considered significant. Secondary outcomes were proportion of relapsing patients during the extension, proportion of patients with CRP > 5mg/l during the extension, cumulative GC dose, DMARD use, EQ-5D score, and adverse events (AE).
Results: The proportion of patients in GC-free remission after one year was significantly higher in the RTX group (48%, 11/23) compared to the PCB group (17%, 4/24), with an absolute difference of 31% (95%-CI 6-56), a relative risk of 2.9 (95%-CI 1.1-7.7), p=0.03. The secondary outcomes showed statistically significant differences in RTX versus PCB in median GC cumulative dose: 1595 versus 2302 mg (p = 0.04) and median PMR-AS: 6 versus 15 (p = 0.02) (
Primary and Secondary Outcomes for Rituximab Versus Placebo Treatment One Year After Infusion
| Placebo [n=24] | Rituximab [n=23] | p-value | |
|---|---|---|---|
| Remission, number (%) | 10 (42%) | 15 (65%) | 0.15 |
| GC-free remission, number (%) | 4 (17%) | 11 (48%) | 0.03 |
| Cumulative GC dose 0-52 weeks, in mg | 2302 (1595 - 2881) | 1595 (1275 – 2260) | 0.04 |
| Cumulative GC dose 21-52 weeks, in mg | 959 (91 – 1442) | 160 (0 - 902) | 0.10 |
| Relapse patient 21-52 weeks, number (%)* | 14 (58%) | 12 (52%) | 0.77 |
| PMR-AS** | 15.25 (7.75 - 22.5) | 6.3 (4.7 - 12.1) | 0.02 |
| CRP serum level, in mg/L | 3.5 (2 - 5) | 3 (1 - 4) | 0.29 |
| physicians’ VAS, 0-10 | 2 (0.2 - 3.7) | 1 (0 - 2) | 0.08 |
| Morning stiffness, in minutes | 25 (4 - 60) | 10 (0 - 30) | 0.06 |
| VAS pain, 0-10 | 3 (1.45 - 6.4) | 1.8 (0.7 - 5) | 0.16 |
| EQ5D-5L, score at week 52# | 0.71 (0.65 – 0.77) | 0.71 (0.63 – 0.77) | 0.87 |
| EQ5D-5L, change week 21-52# | 0 (-0.03 - 0.09) | 0.07 (-0.05 - 0.10) | 0.56 |
| Methotrexate use, number (%) | 4 (17%) | 2 (9%) | 0.67 |
| Adverse events, total, % of patients | 8, 26% | 6, 33% | 0.75 |
Notes . * Relapse was defined as therapy intensification, based on either a) an increase in oral prednisolone, b) adding intramuscular methylprednisolone, or c) starting or switching a DMARD due to treatment inefficiency ** Remission is based on the PMR-AS, calculated by CRP +VASp +VASph + (MST *0.1) + EUL, and a PMR-AS < 10 was considered remission or low disease-activity. # Number of patients for placebo versus RTX were n=23 versus n=23 respectively at week 52, and for comparison of change between week 21-52 total number of patients were n=21 versus n=23 respectively
Cumulative GC Dose 0-52 Weeks, in mg
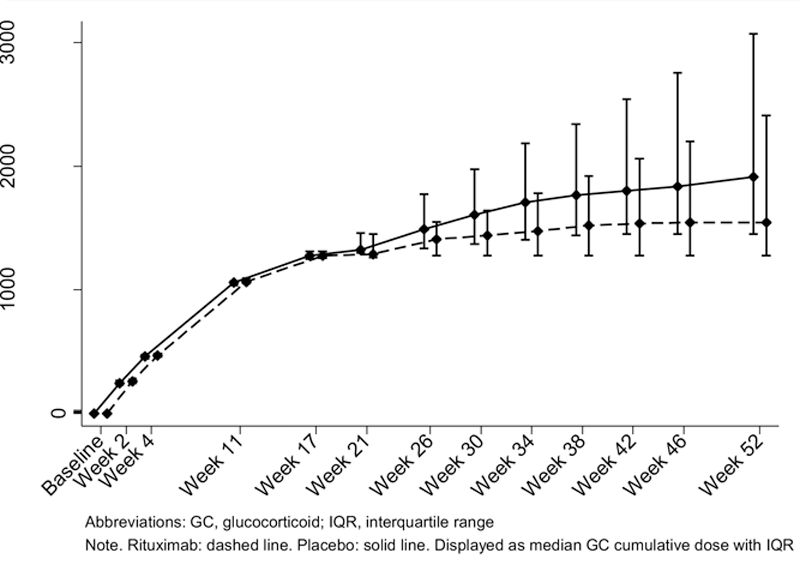
Conclusion: Efficacy of 1x1000 mg RTX in PMR was maintained up to 1 year follow-up, while also demonstrating a GC sparing effect. A larger trial, also assessing effect of on demand retreatment, is needed to confirm our results, and provide insight in which patients most likely benefit from RTX.
REFERENCES:
[1]Dejaco C, et al. Ann Rheum Dis 2015;74(10):1799-807. doi: 10.1136/annrheumdis-2015-207492
[2]Marsman DE, et al. The Lancet Rheumatology 2021;3(11):e758-e66. doi: 10.1016/S2665-9913(21)00245-9
Disclosure of Interests: None declared