

Background: Patients with RA frequently struggle with intolerance of MTX and adherence to MTX remains highly variable. Guidelines conditionally recommend the tapering of MTX before tapering biologic (b)DMARDs, but acknowledge there is an absence of direct evidence. Prior reviews on this topic have focused on tapering of MTX from combination treatment with TNF-inhibitors(i) only 1 . There have been no updated reviews addressing MTX tapering from other targeted therapies such as IL6-i or JAK-i, nor has there been a systematic review addressing this question.
Objectives: To determine the feasibility of tapering MTX to targeted therapy (bDMARDs or JAKi) alone in patients whose RA is controlled (LDA or remission).
Methods: A systematic literature search combing MeSH terms and keywords was conducted in Medline, Embase and Cochrane Library for studies reporting remission outcomes after tapering MTX from targeted therapies in RA. Non-English and animal studies were excluded. Meta-analyses were conducted using random effects models. Forest and funnel plots were created and heterogeneity was calculated.
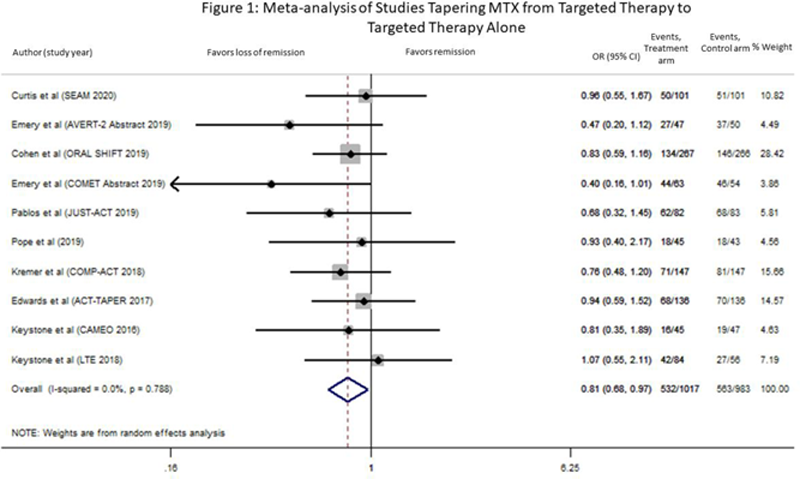
Results: Our search identified 5762 citations. After removal of duplicates and screening title/abstract using the COVIDENCE platform, 504 full-text articles were reviewed. Of the 10 articles meeting our inclusion criteria of tapering MTX to monotherapy with a targeted therapy, 3 studies tapered to etanercept, 3 to tocilizumab, 1 to tofacitinib, 1 to certolizumab pegol, 1 to adalimumab and 1 to abatacept monotherapy. Nine studies were RCTs and one was a long-term extension study (LTE) (
Included Studies
| Author/ Year | n | Early RA | Baseline treatment | MTX Taper Strategy | REM measure | Follow-up |
|---|---|---|---|---|---|---|
| Curtis 2020 | 253 | no | ETA+MTX | Stop | SDAI | 48 wks |
| Emery 2019 | 147 | yes | ABA+MTX | Stop | SDAI | 48 wks |
| Cohen 2019 | 533 | no | TOFA+MTX | Stop | DAS28-CRP | 48 wks |
| Emery 2019 | 411 | yes | ETA+MTX | Taper 4 wks | DAS28 | 52 wks |
| Pablos 2019 | 165 | no | TCZ+MTX | Stop | DAS28 | 28 wks |
| Pope 2019 | 88 | no | CZP+DMARD | Stop | DAS28 | 18 mos |
| Kremer 2018 | 296 | no | TCZ+MTX | Stop | DAS28 | 52 wks |
| Edwards 2017 | 272 | no | TCZ+MTX | Taper 24 wks | DAS28 | 48 wks |
| Keystone 2016 | 205 | no | ETA+MTX | Stop | DAS28 | 18 mos |
| Keystone 2018 | 140 | yes | ADA+MTX | Stop | DAS28-CRP | 3 years |
ETA etanercept, ABA abatacept, TOFA tofacitinib, TCZ tocilizumab, CZP certolizumab pegol, ADA adalimumab, REM remission, wk week, mo month, DAS28 Disease Activity Score 28, SDAI Simplified disease activity index.
Conclusion: Patients with controlled RA have a high probability of maintaining disease control after tapering their MTX to targeted therapy alone, up to 18 months. This review may inform patients with controlled disease on any of a range of targeted therapies and MTX, but who are struggling with MTX-related adverse effects and wish to taper it. Longer follow-up studies with attention to radiographic, functional and patient reported outcomes are needed. The possibility of disease worsening must be discussed with the patient in advance with careful follow-up and prompt re-treatment of disease worsening.
REFERENCES:
[1]Subesinghe S, Scott IC. Expert Rev Clin Pharmacol 2015;8:751-60.
Disclosure of Interests: Charis Meng: None declared, Diviya Rajesh: None declared, Deanna Jannat-Khah Shareholder of: AstraZeneca, Cytodyn, Walgreens, Omar Bruce: None declared, Bridget Jivanelli: None declared, Vivian Bykerk Consultant of: Amgen, Bristol Myers Squibb, Genzyme, Gilead, Janssen, Pfizer, Sanofi-Aventis, UCB., Grant/research support from: NIH (NIAID/NIAMS) grant 1UH2AR067691-01 GRANT11652401 and The Cedar Hill Foundation; institution received grants from Bristol Myers Squibb and Amgen;