

Background: Olokizumab (OKZ) is an interleukin-6-inhibitor for treatment of rheumatoid arthritis (RA). In these analyses we present patient reported outcomes (PROs) reported by TNF-IR patients with moderate to severely active RA receiving OKZ or placebo in a phase 3 randomized controlled trial (RCT) (ClinicalTrials.gov number, NCT02760433).
Objectives: To assess the effect of OKZ treatment compared with placebo in patient global assessment of disease activity (PtGA), pain, physical function (HAQ-DI), fatigue (FACIT-F) and health related quality of life (SF-36 physical (PCS) and mental (MCS) component summary and domain scores) at 12 weeks.
Methods: 368 patients were randomized 2:2:1 to receive subcutaneously administered OKZ 64 mg once every 2 weeks (q2w), OKZ 64 mg q4w, or placebo, plus MTX. PROs were assessed at baseline, weeks 12 (primary endpoint) and 24. At week 16, all patients receiving placebo were switched to either OKZ dose. Between groups differences in least-squares mean (LSM) changes from baseline were analyzed, p < 0.05 considered significant; nominal p-values for PROs not in the hierarchy.
Results: Baseline demographics and disease characteristics were comparable between groups. At week 12, treatment with OKZ q2w compared with placebo resulted in significantly greater LSM changes from baseline in Pain, HAQ-DI, FACIT-F, SF-36 PCS, MCS and 4 domains; with OKZ q4w in PtGA, Pain, SF-36 MCS and 4 domains (
Mean baseline values and LSM changes from baseline to week 12 for PROs
| Baseline, mean (standard deviation) | 12 weeks LSM changes (standard error) | |||||
|---|---|---|---|---|---|---|
| OKZ q2w, N=138 | OKZ q4w, N=161 | Placebo, N=69 | OKZ q2w, N=138 | OKZ q4w, N=161 | Placebo, N=69 | |
| PtGA-VAS (mm) | 64.8 (20.5) | 68.1 (19.1) | 72.1 (18.5) | -24.9 (2.1) | -25.0(1.9)* | -16.9 (2.9) |
| Pain-VAS (mm) | 67.2 (19.5) | 69.3 (19. 1) | 69.6 (21.9) | -28.2 (2.2)** | -27.5(2.0)** | -15.0 (3.0) |
| HAQ-DI† | 1.79 (0.53) | 1.78 (0.56) | 1.78 (0.64) | -0.49 (0.05)* | -0.39(0.04) | -0.32(0.07) |
| SF-36 PCS score | 31.4 (6.8) | 30.6 (7.2) | 30.6 (5.9) | 6.9 (0.7)** | 5.7 (0.6) | 3.9 (0.9) |
| SF-36 MCS score | 44.3 (12.6) | 44.5 (11.1) | 45.1 (10.2) | 4.1 (0.8)* | 3.4 (0.8)* | 0.5 (1.1) |
| Physical functioning | 29.9 (7.9) | 29.8 (8.5) | 29.6 (8.4) | 6.1 (0.8) | 5.2 (0.7) | 3.7 (1.1) |
| Role physical | 32.8 (6.9) | 33.1 (7.4) | 33.7 (6.8) | 6.0 (0.7) | 5.0 (0.7)* | 3.3 (1.0) |
| Bodily pain | 34.5 (6.9) | 33.2 (6.0) | 33.0 (6.6) | 8.5 (0.7)*** | 7.8 (0.7)*** | 3.7 (1.0) |
| General health | 38.3 (8.3) | 36.5 (8.6) | 36.9 (8.5) | 4.7 (0.7)* | 3.3 (0.6) | 2.1 (1.0) |
| Vitality | 40.8 (10.1) | 40.7 (9.5) | 41.1 (8.1) | 5.7 (0.8) | 6.0 (0.7)* | 3.0 (1.1) |
| Social functioning | 38.8 (9.9) | 38.7 (9.8) | 39.6 (9.3) | 6.7 (0.8)*** | 3.6 (0.8)* | 0.2 (1.2) |
| Role emotional | 39.1 (12.5) | 39.1 (11.2) | 38.9 (11.1) | 4.3 (0.9)* | 3.4 (0.8) | 1.0 (1.2) |
| Mental health | 41.4 (11.6) | 41.4 (10.5) | 42.2 (10.3) | 4.4 (0.8) | 4.6 (0.8) | 1.9 (1.1) |
| FACIT-Fatigue | 27.0 (10.2) | 26.6 (10.6) | 27.3 (9.9) | 7.8 (0.9)* | 6.8 (0.8) | 4.6 (1.2) |
Footnotes: NRI for Missing data.
†, secondary endpoint; *p≤0.05, **p<0.01, ***p<0.001 vs placebo;
SF-36 domain changes from baseline to week 12. *p≤0.05, **p<0.01, ***p<0.001 for OKZ q2w vs placebo; *p≤0.05, **p<0.01, ***p<0.001 for OKZ q4w vs placebo; AGNorms, age- and gender-matched normative values; BL, baseline.
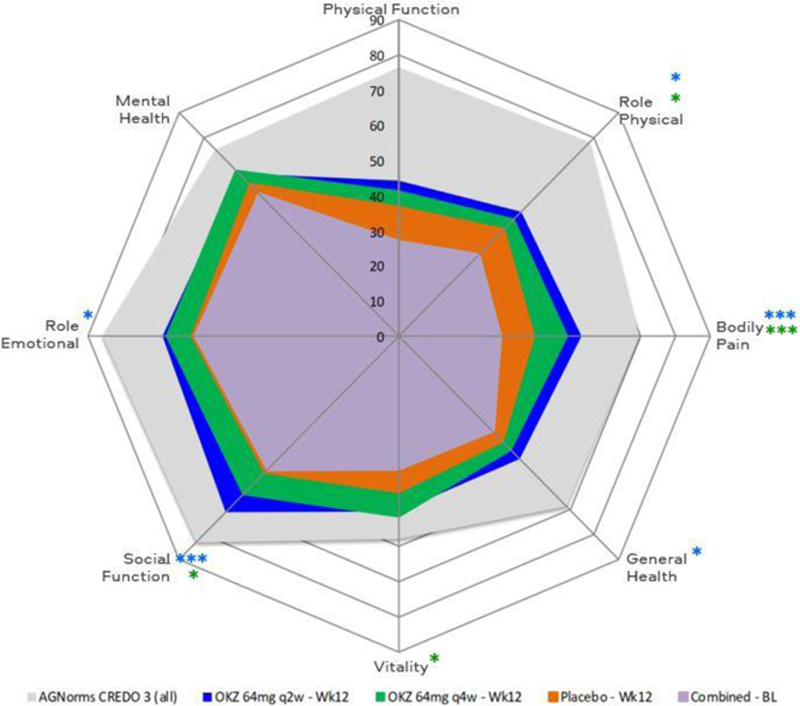
Conclusion: Treatment with OKZ over 12 weeks resulted in statistically significant improvements in PROs vs placebo reported by TNF-IR RA patients. Benefits were more frequently reported by patients receiving OKZ q2w than q4w in this phase 3 RCT of limited size in treatment experienced patients.
Acknowledgements: R-Pharm funded this study; contributed to its design; participated in data collection, analysis, and interpretation of the data; and in the writing, review, and approval of the abstract. No honoraria or payments were made for authorship.
Disclosure of Interests: Vibeke Strand Consultant of: Abbvie, Amgen, Arena, AstraZeneca, Bayer, BMS, Boehringer, Ingelheim, Chemocentryx, Celltrion, Galapagos, Genentech/Roche, Gilead, GSK, Horizon, Inmedix, Janssen, Kiniksa, Lilly, Novartis, Pfizer, Regeneron, Rheos, R-Pharm, Samsung, Sandoz, Sanofi, Scipher, Servier, Setpoint, Sorrento, Spherix, UCB, Ernest Choy Consultant of: Abbvie, Amgen, Bristol Myer Squibbs, Chugai Pharma, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, Regeneron, RPharm, Roche, Sanofi, and UCB., Grant/research support from: Bio-Cancer, Biogen, Novartis, Pfizer, Roche, Sanofi and UCB, Evgeny Nasonov Consultant of: AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, Tatiana Lisitsyna: None declared, Alexander Lila Consultant of: Abbvie, Amgen, Bayer, Biotechnos, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, RPharm, Roche, Sanofi, Stada, Viatris and UCB, Grant/research support from: Novartis, Pfizer, Sofia Kuzkina Employee of: R-Pharm, Mikhail Samsonov Employee of: R-Pharm, Eugen Feist Consultant of: Abbvie, Eli Lilly, Galapagos, Medac, Novartis, Sanofi, Sobi, R-Pharm, Grant/research support from: Eli Lilly, Novartis, Pfizer