

Background: Psoriatic arthritis (PsA) is a burdensome, chronic disease that can impact patient functionality and quality of life. Real-world data are limited regarding the most common disease domain combinations in patients with PsA receiving biologic disease-modifying anti-rheumatic drugs.
Objectives: The objective of this study was to describe PsA disease domain frequency, the most common disease domain combinations of PsA manifestations, and pairwise disease domain prevalence in patients initiating treatment with tumor necrosis factor inhibitors (TNFis) or interleukin-17 (IL-17) inhibitors.
Methods: The CorEvitas PsA/Spondyloarthritis (SpA) Registry is a prospective, observational registry for patients with PsA or SpA under the care of a rheumatologist. The current analysis included adults with PsA who initiated treatment with a TNFi (adalimumab, etanercept, certolizumab pegol, infliximab, golimumab), etanercept (ETN; independent exploratory evaluation as a subset of the TNFi group), or an IL-17 inhibitor (ixekizumab, secukinumab) from January 2013 through December 2020. Baseline disease characteristics among the total population and by therapy group were examined for 6 PsA domains, including enthesitis (ET), dactylitis (DA), peripheral arthritis (PA), nail psoriasis (NP), axial PsA (AX), and skin disease (SD). The top 5 most common domain combinations and frequency of other concomitant disease domains within each domain subpopulation are presented.
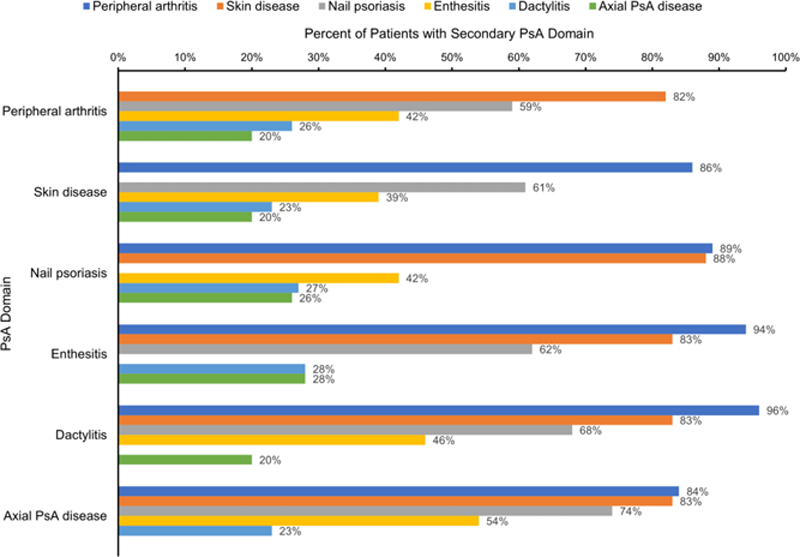
Results: Among 1005 patients initiating treatment for PsA (mean age, 52.9 years; 57% female, 90% white), the prevalence of disease domains was PA (86%), SD (82%), NP (57%), ET (38%), DA (23%), and AX (20%); these proportions were similar among the therapy groups. The frequency of high skin disease (body surface area [BSA] ≥10%) at baseline was highest in IL-17 initiators (23% vs 16% for TNFi and 9% for ETN). TNFi (40%) and ETN (48%) were more frequently observed as first-line therapy compared with IL-17 inhibitors (14%). The most common disease domain combination overall (14%) was PA, NP, and SD; frequency of the top 3 most common domain combinations were similar among therapy groups (
Most common PsA domain combinations overall and by therapy
| Domain Combination Ranking by Frequency, n (%) | Overall (N=1005) | TNFi
| ETN (n=112) | IL-17 (n=374) |
|---|---|---|---|---|
| #1 | PA, NP, SD 138 (14) | PA, NP, SD 91 (14) | PA, SD 17 (15) | PA, NP, SD 47 (13) |
| #2 | PA, SD 122 (12) | PA, SD 84 (13) | ET, PA, NP, SD 13 (12) | ET, PA, NP, SD 41 (11) |
| #3 | ET, PA, NP, SD 95 (9) | ET, PA, NP, SD 54 (9) | PA, NP, SD 12 (11) | PA, SD 38 (10) |
| #4 | ET, PA, SD 64 (6) | DA, PA, NP, SD 38 (6) | SD 7 (6) | ET, PA, SD 33 (9) |
| #5 | DA, PA, NP, SD 61 (6) | ET, PA, SD 31 (5) | ET, PA, NP, AX, SD 6 (5) | ET, PA, NP, AX, SD 24 (6) |
Matching domain combinations are shaded across each therapy group.
ET, enthesitis; DA, dactylitis; PA, peripheral arthritis; NP, nail psoriasis; AX, axial PsA; SD, skin disease; TNFi, tumor necrosis factor inhibitors; ETN, etanercept; IL-17, interleukin-17 inhibitors.
a TNFi includes ETN initiators.
Conclusion: The most common disease domains and domain combinations were similar among initiators of TNFis, ETN, and IL-17s. IL-17 initiators had high skin disease (BSA ≥10%) more often and initiated as first-line therapy less frequently than TNFi initiators. Assessing all PsA domains is important for optimal disease management.
Acknowledgements: This study is sponsored by CorEvitas, LLC. CorEvitas has been supported through contracted subscriptions in the last two years by AbbVie, Amgen Inc., Arena, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chugai, Eli Lilly and Company, Genentech, Gilead, GSK, Janssen, LEO, Novartis, Ortho Dermatologics, Pfizer Inc., Regeneron, Sanofi, Sun, and UCB. Writing support was funded by Amgen Inc. and provided by Jacob Huffman, PhD of Peloton Advantage, LLC, an OPEN Health company, and Julie Wang, DPM, of Amgen Inc.
Disclosure of Interests: Philip J Mease Speakers bureau: AbbVie, Amgen Inc., Eli Lilly, Janssen, Novartis, Pfizer, and UCB – speakers bureau, Consultant of: AbbVie, Amgen Inc., Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, GlaxoSmithKline, Novartis, Pfizer, Sun, and UCB – grant/research support and consultant, Grant/research support from: AbbVie, Amgen Inc., Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, GlaxoSmithKline, Novartis, Pfizer, Sun, and UCB – grant/research support and consultant, Taylor Blachley Employee of: CorEvitas, LLC – employment, Jacqueline O’Brien Employee of: CorEvitas, LLC – employment, Nicole Middaugh Employee of: CorEvitas, LLC – employment, Greg Kricorian Shareholder of: Amgen Inc. – employment and stock ownership, Employee of: Amgen Inc. – employment and stock ownership, Scott Stryker Shareholder of: Amgen Inc. – employment and stock ownership, Employee of: Amgen Inc. – employment and stock ownership, David Collier Shareholder of: Amgen Inc. – employment and stock ownership, Employee of: Amgen Inc. – employment and stock ownership, Alexis Ogdie Shareholder of: Royalties to husband from Novartis, Consultant of: AbbVie, Amgen Inc., Bristol Myers Squibb, Celgene, CorEvitas’ Psoriatic Arthritis/Spondyloarthritis Registry (formerly Corrona), Eli Lilly, Gilead, Janssen, Novartis, Pfizer, and UCB – consultant, Grant/research support from: AbbVie, Amgen Inc., Novartis, and Pfizer – grant/research support