

Background: For children with polyarticular juvenile idiopathic arthritis (pJIA) and inadequate response or intolerance to initial treatment with MTX, treatment options include abatacept. 1 Abatacept, a selective T-cell co-stimulation modulator, has a distinct mechanism of action from other current treatments for rheumatic diseases, 2 and factors predicting clinical response can help determine optimal treatment strategy. Two phase 3 studies demonstrated the efficacy and safety of IV and SC abatacept in patients with pJIA and an inadequate response to other DMARDs. 2,3
Objectives: To determine baseline and post-baseline factors that may predict a clinical response in children and adolescents with pJIA treated with abatacept for 2 years.
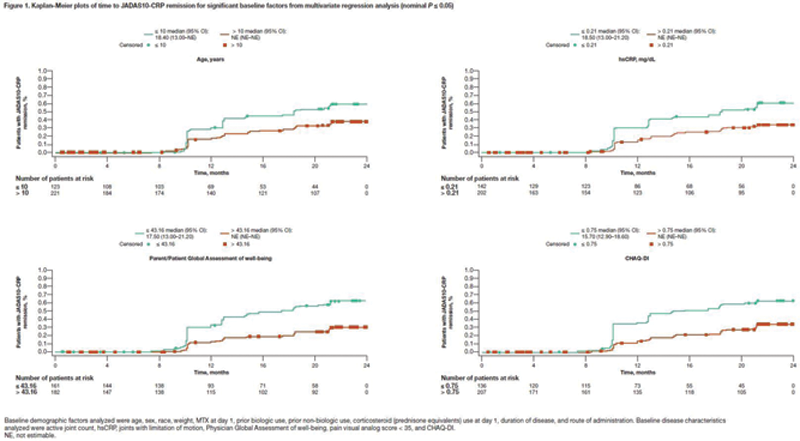
Methods: Baseline demographic and disease characteristics and post-baseline factors (50% and 70% improvement in ACR criteria [ACR50, ACR70] at days 57 and 85) were analyzed using data from 2 phase 3 studies of abatacept in patients with JIA aged 2–17 years (SC administration) and 6–17 years (IV administration). Efficacy endpoints were Juvenile Arthritis Disease Activity Score in 10 joints based on CRP (JADAS10-CRP) inactive disease (ID; score of ≤ 2.7), 4 and remission, defined as 6 consecutive months of post-baseline JADAS10-CRP ID. Data were analyzed over the entire 2-year study period. The earliest time point at which patients achieved these outcomes was reported. The aforementioned study factors were subjected to a time-to-event analysis, including Cox proportional hazards univariate regression analysis and Cox proportional hazards multivariate regression analysis using stepwise regression; results of the multivariate analysis are reported. Kaplan–Meier analysis was used to estimate time to achieve clinical response. Receiver operating characteristic curves were used to determine threshold values for continuous variables.
Results: Overall, 347 patients were included in the analysis (SC, n = 219; IV, n = 128; 73.8% female; mean [SD] age, 11.3 [4.0] years). Following abatacept treatment, both time to JADAS10-CRP ID and time to JADAS10-CRP remission were predicted (nominal
P
≤ 0.05) by age (≤ 11 years: hazard ratio [HR], 1.52 [95% CI, 1.14–2.02] and ≤ 10 years: HR, 1.73 [95% CI, 1.20–2.48], respectively), high-sensitivity CRP (hsCRP; ≤ 0.6 mg/dL: HR, 1.67 [95% CI, 1.22–2.28] and ≤ 0.21 mg/dL: HR, 1.67 [95% CI, 1.15–2.42], respectively), Parent/Patient Global Assessment of well-being (≤ 35.86: HR, 1.88 [95% CI, 1.41–2.51] and ≤ 43.16: HR, 2.05 [95% CI, 1.35–3.10], respectively), and Childhood HAQ-DI (CHAQ-DI; ≤ 1.63: HR, 2.23 [95% CI, 1.47–3.39] and ≤ 0.75: HR, 1.84 [95% CI, 1.24–2.73], respectively) (remission data shown in
Conclusion: We identified baseline and post-baseline factors that predicted JADAS10-CRP ID and remission in patients with pJIA treated with abatacept for 2 years. Screening of abatacept-treated patients with pJIA for such factors may help predict earlier achievement of ID and/or remission.
REFERENCES:
[1]Ringold S, et al. Arthritis Rheumatol 2019;71:846–63.
[2]Brunner HI, et al. Arthritis Rheumatol 2018;70:1144–54.
[3]Ruperto N, et al. Lancet 2008;372:383–91.
[4]Trincianti C, et al. Arthritis Rheumatol 2021;73:1966–75.
Acknowledgements: This study was sponsored by Bristol Myers Squibb. Writing and editorial assistance were provided by Candice Judith Dcosta, MSc, of Caudex, funded by Bristol Myers Squibb. We would like to acknowledge Mara Becker, Duke Clinical Research Institute, Durham, NC, USA, for her contribution to the study analysis.
Disclosure of Interests: Nicolino Ruperto Speakers bureau: Honoraria for consultancies or speaker bureaus from the following pharmaceutical companies in the past 3 years: 2 Bridge, Amgen, AstraZeneca, Aurinia, Bayer, Brystol Myers Squibb, Cambridge Healthcare Research, Celgene, Domain Therapeutic, Eli Lilly, EMD Serono, GlaxoSmithKline, Idorsia, inMed, Janssen, Novartis, Pfizer, Sobi, UCB, Consultant of: Honoraria for consultancies or speaker bureaus from the following pharmaceutical companies in the past 3 years: 2 Bridge, Amgen, AstraZeneca, Aurinia, Bayer, Brystol Myers Squibb, Cambridge Healthcare Research, Celgene, Domain Therapeutic, Eli Lilly, EMD Serono, GlaxoSmithKline, Idorsia, inMed, Janssen, Novartis, Pfizer, Sobi, UCB, Hermine Brunner Speakers bureau: GlaxoSmithKline, Novartis, Pfizer, Consultant of: AbbVie, AstraZeneca-Medimmune, Biogen, Boehringer, Bristol Myers Squibb, Celgene, Cerocor, Eli Lilly, EMD Serono, F. Hoffmann-La Roche, GlaxoSmithKline, Idorsia, Janssen, Merck, Novartis, R-Pharm, Sanofi, Grant/research support from: The Cincinnati Children’s Hospital, where HIB works as a full-time public employee, has received contributions from the following industries in the past 3 years: Bristol Myers Squibb, F. Hoffmann-La Roche, Janssen, Novartis, and Pfizer. This funding has been reinvested for the research activities of the hospital in a fully independent manner, without any commitment to third parties, Alberto Berman Grant/research support from: AbbVie, Amgen, Bristol Myers Squibb, Lilly, Novartis, Pfizer, Roche, Francisco Avila Zapata: None declared, Gerd Horneff Speakers bureau: AbbVie, Chugai, Janssen, Novartis, Pfizer, Grant/research support from: AbbVie, Chugai, MSD, Novartis, Pfizer, Roche, Linda Wagner-Weiner Grant/research support from: Abbott, Bristol Myers Squibb, Merck, Pfizer, UCB, Alexander Belot Speakers bureau: Chugai, GlaxoSmithKline, Novartis, Roche (punctual scientific intervention), Grant/research support from: Boehringer Ingelheim, Merck (joint research project), Ruben Burgos-Vargas: None declared, Maria Luz Gámir Gámir: None declared, Claudia Goldenstein-Schainberg Speakers bureau: AbbVie, Janssen, Novartis, Consultant of: AbbVie, Janssen, Novartis, Maria T. Terreri: None declared, Margarita Askelson Consultant of: Acerta Pharma, Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Robert Wong Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Alberto Martini Consultant of: AbbVie, Eli Lilly, EMD Serono, Idorsia, Janssen, Novartis, Pfizer, Daniel J Lovell Consultant of: AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Hoffman LaRoche, Novartis, UBC (all contracts with employer, CCHMC), Grant/research support from: Bristol Myers Squibb, Janssen, Pfizer, Roche (all contracts with employer, CCHMC); NIH grants: NIAMS, NICHD