

Background: Rheumatoid arthritis (RA) is the most common systemic autoimmune disease that primarily affects joints but is also often characterized by extra-articular involvement 1 . Cardiovascular diseases are the most important causes of sudden death in these patients, which present a risk of developing cardiovascular events increased by 48% 2 . The causes of increased cardiovascular risk are several and not completely understood, but recent evidence supports the key role of endothelial dysfunction in pathogenesis. In this complex scenario, it is known that IL-6 receptors are present at the endothelial level and can be activated leading to endothelial dysfunction. SARS-Cov-2 is a coronavirus responsible for the disease called ‘coronavirus disease 2019’ (CoViD-19) characterized by clinical manifestations ranging from a flu-like syndrome up to severe lung damage associated with systemic hyper cytokine syndrome that can lead to multiple organ failure and death. Therefore, both RA and Covid-19 are associated with an increased pro-thrombotic and cardiovascular risk and IL-6 might be crucial in the pathophysiological mechanisms of both diseases.
Objectives: The main hypothesis of this study was to evaluate the possible role of IL-6 as a promoter of endothelial dysfunction in RA and CoViD-19.
Methods: In vitro experiments were conducted on the endothelial cell line EA. hy926. Cells were treated for 24 h with fetal bovine serum (FBS), a pool of RA patients’ sera or a pool of CoViD-19 patients’ sera. The expression levels of adhesion molecules (V-CAM1/CD-106, I-CAM/CD-54, p-selectine/CD-62, tissue factor/CD-142) and apoptosis were analyzed using cytofluorimetric technique. In addition, the autophagy level, using the autophagy markers p62 and LC3II, were evaluated through a western-blot analysis. The same experiments were conducted co-treating cells with the same pool of sera in addition to tocilizumab (TCZ), an anti-IL-6 drug, to verify the reversibility of the process and test the role of the aforementioned cytokine. Data are reported as interquartile median values. The Kruskal Wallis test was used for unpaired samples and the Mann-Whitney test for paired samples. P<0.05 values were considered statistically significant.
Results: EA. hy926 cells, when treated with both RA and CoViD-19 patients’ sera, showed increased levels of activation molecules and apoptosis compared to FBS treated cells. In addition, we observed increased levels of both p62 and LC3 proteins after both rheumatoid arthritis and CoViD-19 patients’ sera treatment. All these findings were reversible in the presence of TCZ. The results are presented in
Figures show the adhesion molecules levels ( A ), apoptosis levels ( B ), p62 and LC3II levels ( C ), in all experimental conditions. FBS 10% (cells treated with FBS at 10% concentration), S AR (cells treated with a pool of RA patients’ sera); S Covid (cells treated with a pool of COVID-19 patients’ sera); FBS 10%+toci (cells co-treated with FBS at 10% concentration and TCZ); S AR+toci (cells co-treated with a pool of RA patients’ sera and TCZ); S Covid+toci (cells co-treated with a pool of CoViD-19 patients’ sera and TCZ).
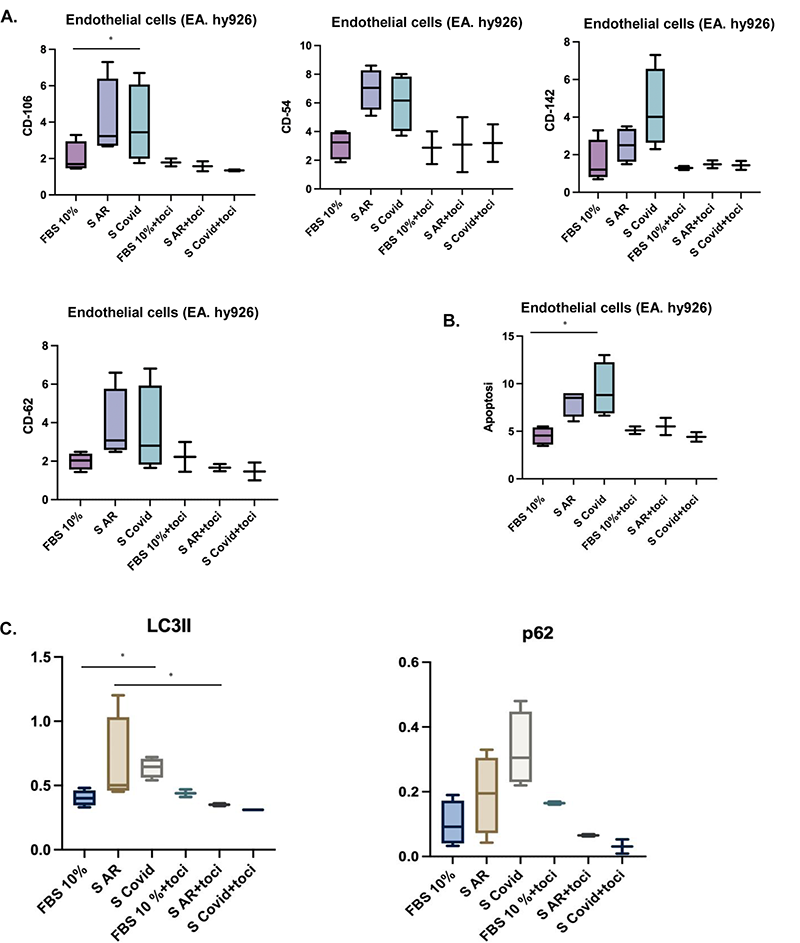
Conclusion: Our data showed that treatment with RA and CoViD-19 patients’ sera increase the activation and death of endothelial cells in vitro. The increased level of cells death is possibly due to a block of autophagy. The reversibility of the process after blocking IL-6 with TCZ co-treatment confirms the hypothesis that IL-6 can play a key role in the pathogenesis of endothelial damage in patients with RA and CoViD-19.
REFERENCES:
[1]Bordy R et al. Microvascular endothelial dysfunction in rheumatoid arthritis. Nat Rev Rheumatol. 2018 Jul;14(7):404-420.
[2]Avina-Zubieta et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann. Rheum. Dis. 2012; 71:1524–1529.
Disclosure of Interests: None declared.