

Background: A treat-to-target approach for RA management is recommended. 1,2 However, up to half of patients discontinue DMARD treatment within 18 months. 2 Predictive biomarkers, such as anti-citrullinated protein antibodies (ACPAs) and RF, may be useful to stratify patients to the most appropriate treatment. ACTION ( A bata C ep T I n r O uti N e clinical practice; NCT02109666) and ASCORE ( A batacept S ub C utane O us in R outine Clinical Practic E ; NCT02090556) were 2-year, international, observational, prospective, multicenter studies of IV and SC abatacept, respectively, for the treatment of RA in routine clinical practice. 3,4 Higher retention has been previously reported in patients with double ACPA/RF seropositive RA compared with double ACPA/RF seronegative RA. 3,4
Objectives: To assess the independent effect of ACPA or RF single seropositivity on abatacept retention in patients with RA receiving abatacept in a post hoc analysis of ACTION and ASCORE.
Methods: This post hoc analysis included patients aged ≥ 18 years, with active moderate-to-severe RA (ACR/EULAR 2010 criteria) who initiated IV (body weight–adjusted dosing) or SC (125 mg once weekly) abatacept. 3,4 Patients were stratified by baseline ACPA/RF status: ACPA+/RF− (ACPA+ only), ACPA/RF double positive (+/+), ACPA−/RF+ (RF+ only), and ACPA/RF double negative (−/−). Abatacept retention rate at 2 years was estimated by Kaplan–Meier (KM) analysis.
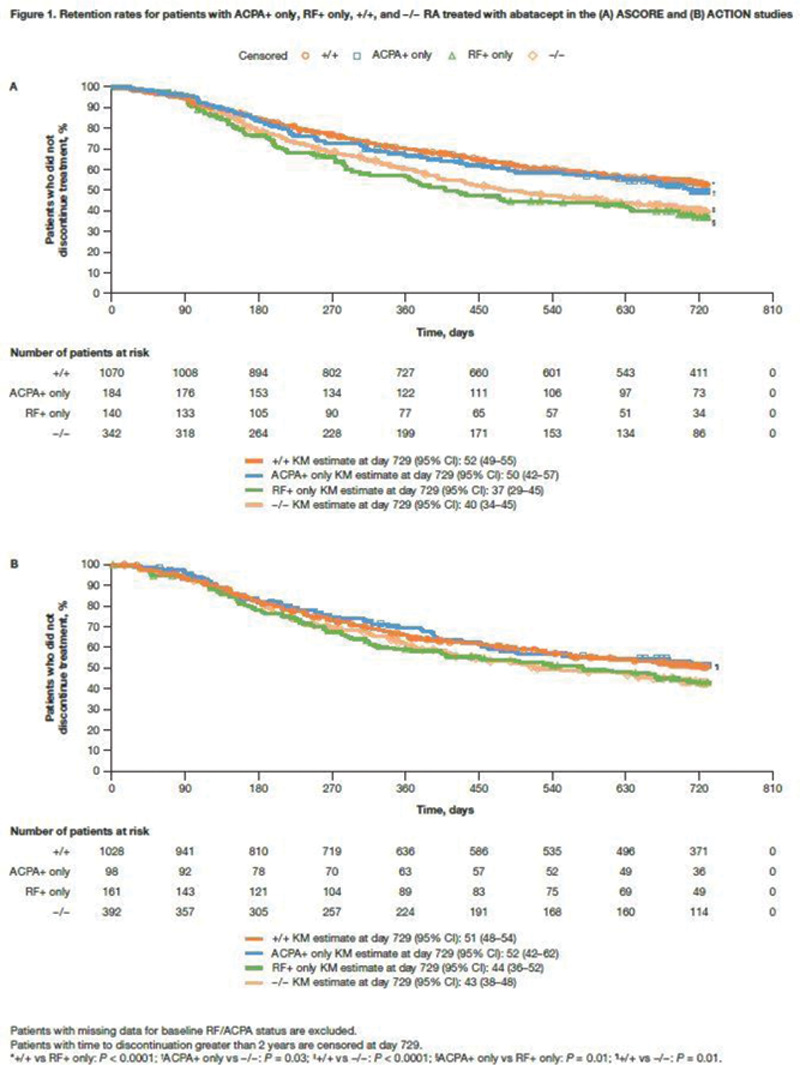
Results: Patients with ACPA/RF serostatus data from the ACTION and ASCORE studies (N = 1679 and N = 1748, respectively) were evaluated. Baseline demographic and disease characteristics were similar across studies and serostatus groups (
Baseline demographics and disease characteristics by ACPA/RF status for the ASCORE and ACTION studies
| ASCORE | ||||
|---|---|---|---|---|
| +/+ | RF+ only | ACPA+ only | −/− | |
| (n = 1079 ) | (n = 142 ) | (n = 184 ) | (n = 343 ) | |
| Age, years | 57.1 (12.8) | 58.2 (11.8) | 57.4 (13.5) | 57.8 (13.9) |
| DAS28 (CRP ) | 4.7 (1.2) | 4.6 (1.1) | 4.4 (1.0) | 4.8 (1.2) |
| CDAI | 26.6 (12.5) | 25.8 (12.0) | 23.6 (10.9) | 28.2 (13.2) |
| SDAI | 28.1 (13.0) | 27.2 (12.4) | 24.4 (10.8) | 29.7 (13.9) |
| ACTION | ||||
| +/+ | RF+ only | ACPA+ only | −/− | |
| (n = 1028 ) | (n = 161 ) | (n = 98 ) | (n = 392 ) | |
| Age, years | 58.2 (12.0) | 58.4 (13.4) | 58.5 (14.0) | 57.0 (13.3) |
| DAS28 (CRP ) | 4.9 (1.1) | 5.0 (1.1) | 4.9 (1.0) | 5.0 (1.1) |
| CDAI | 28.7 (12.2) | 29.2 (12.4) | 28.7 (11.5) | 30.1 (12.9) |
| SDAI | 30.4 (13.1) | 31.2 (13.4) | 29.8 (11.5) | 31.7 (13.4) |
Data are mean (SD). Patients with missing data for baseline ACPA/RF status are excluded.
Conclusion: In this post hoc analysis of the real-world ACTION and ASCORE studies, ACPA positivity was associated with an increased likelihood of retention over 2 years. Patients with ACPA+ only RA were equally as likely to be retained on abatacept as patients with ACPA/RF double positivity. In contrast, patients with RF+ only RA were less likely to be retained on abatacept over 2 years. These findings suggest that ACPA positivity played a more important role than RF positivity in abatacept retention. The higher retention seen in patients with ACPA+ only vs RF+ only disease demonstrates the key role of ACPA in RA and supports the importance of precision medicine in treating patients.
REFERENCES:
[1]Fraenkel L, et al. Arthritis Care Res (Hoboken ) 2021;73:924–39.
[2]Smolen JS, et al. Ann Rheum Dis 2020;79:685–99.
[3]Alten R, et al. Clin Rheumatol 2019;38:1413–24.
[4]Alten R, et al. Ann Rheum Dis 2021;80(suppl 1):OP0180.
Acknowledgements: This study was sponsored by Bristol Myers Squibb. Medical writing and editorial assistance was provided by Fiona Boswell, PhD, of Caudex, and was funded by Bristol Myers Squibb. Study management provided by Syneos (CRO).
Disclosure of Interests: Rieke Alten Speakers bureau: AbbVie, Bristol Myers Squibb, Celltrion, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Paid instructor for: AbbVie, Bristol Myers Squibb, Celltrion, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Consultant of: AbbVie, Bristol Myers Squibb, Celltrion, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Grant/research support from: AbbVie, Bristol Myers Squibb, Celltrion, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Christiane Rauch Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Melanie Chartier Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, M.T. Nurmohamed Speakers bureau: AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen, Pfizer, Consultant of: AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen, Pfizer, Grant/research support from: AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Janssen, MSD, Pfizer, Sean Connolly Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Maya H Buch Speakers bureau: AbbVie, Consultant of: AbbVie, Galapagos, Gilead, Pfizer, Grant/research support from: Gilead, Pfizer, UCB, Peter Peichl Speakers bureau: GlaxoSmithKline, Janssen, Xavier Mariette Consultant of: Bristol Myers Squibb, Galapagos, GlaxoSmithKline, Janssen, Novartis, Pfizer, Sanofi, UCB, Yusuf Patel: None declared, Sara Marsal Speakers bureau: Bristol Myers Squibb, Lilly, MSD, Novartis - Sandoz, Pfizer, Roche, Consultant of: AbbVie, Galapagos, Pfizer, Sanofi; IMIDomics (executive role), Grant/research support from: AbbVie, Bristol Myers Squibb, Galapagos, Janssen, Lilly, MSD, Novartis - Sandoz, Pfizer, Roche, Sanofi, UCB, Roberto Caporali Speakers bureau: AbbVie, Amgen, Bristol Myers Squibb, Celltrion, Fresenius-Kabi, Galapagos, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, UCB, Consultant of: AbbVie, Amgen, Bristol Myers Squibb, Celltrion, Galapagos, Janssen, Lilly, MSD, Novartis, Pfizer, Sandoz, UCB, Hedley Griffiths Consultant of: Amgen, Raimón Sanmartí Speakers bureau: AbbVie, Bristol Myers Squibb, Lilly, MSD, Pfizer, Roche, Sanofi, Grant/research support from: AbbVie, Bristol Myers Squibb, MSD, Pfizer, Roche, Bettina Bannert Speakers bureau: Novartis Pharma Schweiz AG, Yedid Elbez Consultant of: Bristol Myers Squibb, Employee of: Signifience, Karissa Lozenski Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb.