

Background: Plasma calprotectin is a sensitive inflammatory marker in patients with rheumatoid arthritis (RA) and reflects activation of granulocytes and macrophages. Plasma calprotectin has not previously been studied in a head-to-head trial of multiple biological mechanisms of action versus active conventional therapy (ACT) with methotrexate and prednisolone.
Objectives: To assess the effect of treatment on plasma calprotectin levels in patients with early RA by determining the 24-week change in the four arms of the NORD-STAR Study, a large multicenter randomized head-to-head clinical trial of ACT versus tumor necrosis factor inhibition, T-cell co-stimulation inhibition, and interleukin-6 inhibition (1).
Methods: Calprotectin was analyzed in plasma samples at baseline, week 4 and week 24 from 400 treatment naïve patients with early RA in the NORD-STAR Study. Samples were analyzed using a calprotectin ELISA alkaline phosphatase (ALP) kit from CalproLab (Oslo, Norway) in a Dynex DS2 processing system (normal levels <910 µg/L). Patients were assessed by clinical (CRP, 28 SJC/TJC, physician global) and patients’ reported assessments. Crude and adjusted linear regression analyses were performed in R 4.0.3 with calprotectin levels at week 24 as the outcome. The four arms were represented by three dummy variables. The adjustment variables were age, sex, anti-CCP status and country. Both analyses were adjusted for baseline calprotectin levels.
Results: At baseline, the mean time since diagnosis was 15.7 days (SD) (22.9), mean age 53.7 (15.0) years, ACPA positive 81%, and female 66%. Mean calprotectin levels were 1931 (1495) µg/L at baseline, 866 (951) µg/L at week 4, and 629 (661) µg/L at week 24. At baseline, normal calprotectin levels (<910 µg/L) were observed in 27% of all patients (ACT 22%, certolizumab-pegol and methotrexate 30%, abatacept and methotrexate 25%, tocilizumab and methotrexate 31%). At week 24, normal calprotectin levels were observed in 82% of all patients (ACT 68%, certolizumab-pegol and methotrexate 91%, abatacept and methotrexate 80%, tocilizumab and methotrexate 90%).
Observed calprotectin levels at week 24 were significantly lower in patients treated with certolizumab-pegol and methotrexate -336µg/L (97) (p< 0.006) or tocilizumab and methotrexate -284 (99) (p < 0.004), versus ACT when adjusted for age, sex, anti-CCP status, baseline calprotectin level, and country; however, a significant difference was not observed in patients treated with abatacept and methotrexate -110 (96) (p = 0.25). The
Average percentage change in calprotectin levels from baseline to week 24. ACT: active conventional therapy, CZP+MTX: certolizumab-pegol and methotrexate, ABA+MTX: abatacept and methotrexate, TCZ+MTX: tocilizumab and methotrexate.
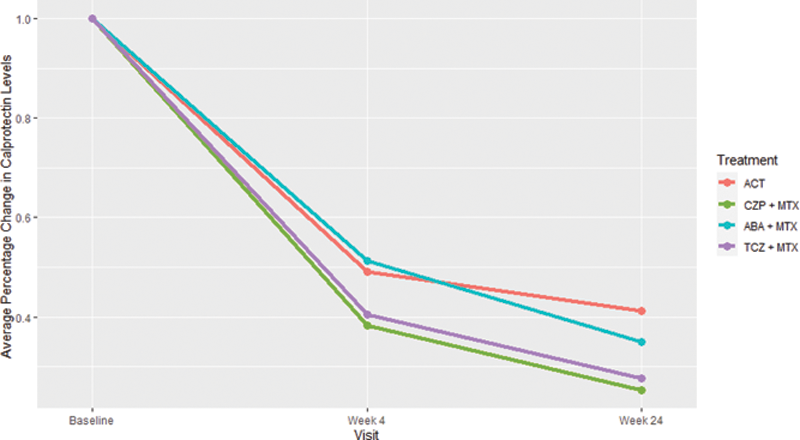
Conclusion: Calprotectin, a sensitive biomarker of inflammation, normalized in the majority of patients. The decline differed between treatment groups and was largest in patients treated with a TNF inhibitor and methotrexate, suggesting that calprotectin reflects the activity of specific inflammatory pathways rather than overall inflammation. The findings of this study should be further explored.
REFERENCES:
[1]Hetland ML, et. al., Active conventional treatment and three different biological treatments in early rheumatoid arthritis: phase IV investigator initiated, randomised, observer blinded clinical trial. BMJ. 2020 Dec 2;371:m4328. doi: 10.1136/bmj.m4328. PMID: 33268527; PMCID: PMC7708829.
Acknowledgements: I would like to acknowledge the NORD-STAR Study group.
Disclosure of Interests: David Stevens: None declared, Marte Heiberg: None declared, Amirhossein Kazemi: None declared, Ronald van Vollenhoven: None declared, Jon Lampa: None declared, Anna Rudin: None declared, Kristina Lend: None declared, Merete Lund Hetland: None declared, Mikkel Østergaard: None declared, Michael Nurmohamed: None declared, Kim Hørslev-Petersen: None declared, Dan Nordström Consultant of: Abbvie, BMS, Lilly, MSD, Novartis, Pfizer, Roche and UCB, Björn Gudbjornsson: None declared, Till Uhlig: None declared, Espen A Haavardsholm: None declared, Hilde Berner Hammer Speakers bureau: AbbVie, Novartis, and Lilly.