

Background: The prevalence of rheumatoid arthritis (RA) in persons 60 years or older is estimated to be 2%. Late onset rheumatoid arthritis (LORA) is a sub-group of patients traditionally defined as onset of RA after the age of 60 years. Compared to younger onset rheumatoid arthritis (YORA) which occurs before the age of 60 years, LORA has unique characteristics and disease manifestations. The prognosis of LORA patients is less clear based on prior studies.
Objectives: We compared the clinical characteristics, time to remission and treatment regimen at remission between LORA and YORA patients.
Methods: The Ontario Best Practices Research Initiative (OBRI) is a clinical registry of RA patients followed in routine care. This analysis used the OBRI database from 2008 to 2020. Patients were included if they had active RA disease (≥1 swollen joint) and were enrolled in the study within 1 year of diagnosis. LORA was defined as diagnosis of RA after age of 60, YORA as under age of 60. Remission was defined by Disease Activity Score 28 (DAS28) ≤2.6. A multivariable Cox proportional hazards model was used to estimate time to remission.
Results: The Ontario Best Practices Research Initiative (OBRI) is a clinical registry of RA patients followed in routine care. This analysis used the OBRI database from 2008 to 2020. Patients were included if they had active RA disease (≥1 swollen joint) and were enrolled in the study within 1 year of diagnosis. LORA was defined as diagnosis of RA after age of 60, YORA as under age of 60. Remission was defined by Disease Activity Score 28 (DAS28) ≤2.6. A multivariable Cox proportional hazards model was used to estimate time to remission.
Cox proportional hazards model predicting time to remission
| Baseline characteristics | Univariate | p-value | Multivariable | p-value |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Sociodemographic | ||||
| Female gender | 0.71 (0.60-0.84) | <.0001 | 0.87 (0.70-1.09) | 0.2256 |
| Post-secondary education | 1.26 (1.08-1.47) | 0.0039 | 1.04 (0.87-0.70) | 0.6744 |
| Ever smoked | 0.87 (0.75-1.02) | 0.076 | 0.93 (0.77-1.12) | 0.4269 |
| RA family history | 0.89 (0.74-1.07) | 0.2176 | 0.87 (0.70-1.70) | 0.1817 |
| Disease characteristics | ||||
| Positive rheumatoid factor | 1.01 (0.85-1.19) | 0.9182 | 0.94 (0.78-1.14) | 0.5381 |
| * HAQ-DI | 0.62 (0.55-0.69) | <.0001 | 0.71 (0.61-0.84) | <.0001 |
| Morning stiffness (>30 mins) | 0.71 (0.61-0.83) | <.0001 | 0.89 (0.73-1.08) | 0.2366 |
| Joint erosion | 0.94 (0.77-1.14) | 0.5224 | 0.87 (0.70-1.08) | 0.1954 |
| DAS28 | 0.77 (0.72-0.82) | <.0001 | 0.88 (0.80-0.96) | 0.0048 |
| Number of comorbidities | 0.83 (0.77-0.88) | <.0001 | 0.88 (0.81-0.95) | 0.0019 |
| Treatment | ||||
| Biologic or JAK inhibitor (time variant) | 0.86 (0.71-1.03) | 0.09 | 1.53 (0.63-3.69) | 0.3485 |
| LORA | 0.83 (0.71-0.97) | 0.0194 | 1.10 (0.90-1.34) | 0.3593 |
* HAQ-DI = health assessment questionnaire disability index
Kaplan Meier survival curve of time to remission.
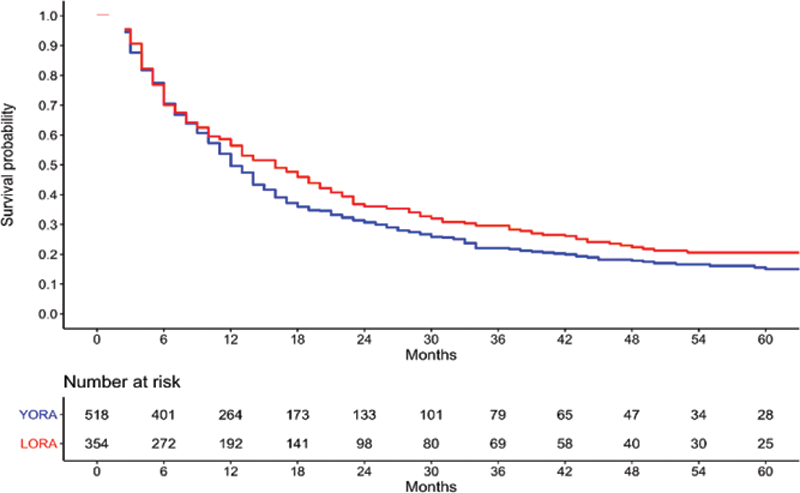
Conclusion: LORA and YORA patients had similar prognosis in terms of time to remission. At remission, LORA patients were more likely to be on a single csDMARD without biologic or JAK inhibitor. Clinicians should take the same approach for all RA patients targeting remission regardless of age of onset.
REFERENCES:
[1]Ruban TN, Jacob B, Pope JE, Keystone EC, Bombardier C, Kuriya B. The influence of age at disease onset on disease activity and disability: results from the Ontario Best Practices Research Initiative. Clinical rheumatology. 2016 Mar;35(3):759-63
Disclosure of Interests: Xiuying Li Grant/research support from: OBRI was funded by peer reviewed grants from CIHR (Canadian Institute for Health Research), Ontario Ministry of Health and Long-Term Care (MOHLTC), Canadian Arthritis Network (CAN) and unrestricted grants from: Abbvie, Amgen, Janssen, Medexus, Merck, Novartis, and Pfizer, Angela Cesta Grant/research support from: The OBRI registry is funded by peer reviewed grants from CIHR (Canadian Institute for Health Research), Ontario Ministry of Health and Long-Term Care (MOHLTC), Canadian Arthritis Network (CAN) and unrestricted grants from: AbbVie, Amgen, Janssen, Merck, Novartis, and Pfizer, Mohammad Movahedi Grant/research support from: The OBRI registry is funded by peer reviewed grants from CIHR (Canadian Institute for Health Research), Ontario Ministry of Health and Long-Term Care (MOHLTC), Canadian Arthritis Network (CAN) and unrestricted grants from: AbbVie, Amgen, Janssen, Merck, Novartis, and Pfizer, Claire Bombardier Grant/research support from: The OBRI registry is funded by peer reviewed grants from CIHR (Canadian Institute for Health Research), Ontario Ministry of Health and Long-Term Care (MOHLTC), Canadian Arthritis Network (CAN) and unrestricted grants from: AbbVie, Amgen, Janssen, Merck, Novartis, and Pfizer.