

Background: Patients (pts) with rheumatoid arthritis (RA) are often administered glucocorticoids (GCs) as bridging therapy when initiating or adjusting disease-modifying antirheumatic drugs (DMARDs). Due to their systemic effects, short-term use of GCs at the lowest possible dose is recommended with rapid tapering. 1
Objectives: We describe GC discontinuation patterns and the associated clinical outcomes in pts with RA receiving upadacitinib (UPA) or adalimumab (ADA).
Methods: SELECT-COMPARE is a randomized phase 3 trial of UPA vs placebo and ADA with a 48-week (wk) double-blind treatment period and a 10-year long-term extension in pts with RA receiving concomitant methotrexate (MTX) who had an inadequate response to MTX. 2 Background GCs (≤10 mg/day prednisone or equivalent) were permitted and could be tapered or discontinued starting at wk 26 per physician discretion. This post hoc analysis included pts who received ≥1 dose of UPA 15 mg once daily or ADA 40 mg every other wk while on concomitant GCs at baseline. The proportion of pts with disease worsening (Clinical Disease Activity Index [CDAI] >2 and Disease Activity Score 28-joint count C-reactive protein [DAS28-CRP] >0.6) following GC discontinuation through follow-up is described. Maintenance of clinical response, including remission and low disease activity based on CDAI ≤2.8 and ≤10, respectively, as well as DAS28-CRP <2.6 and ≤3.2, were assessed among pts who discontinued GCs. Adverse events (AEs) were assessed before and after GC discontinuation through follow-up. Data were analyzed descriptively.
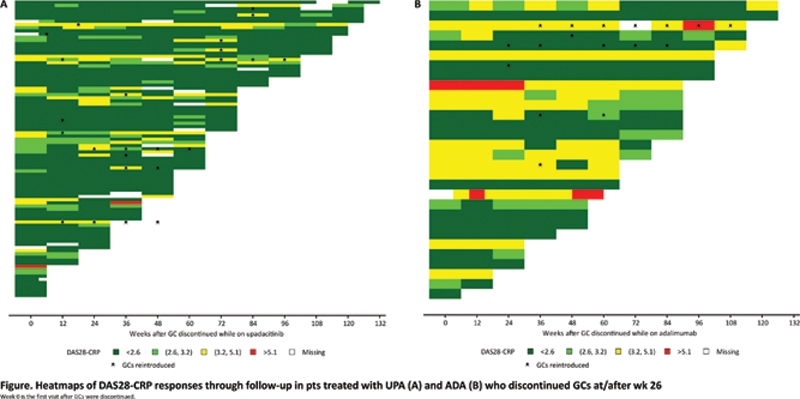
Results: Of 1,629 pts randomized, 978 (60%) used GCs at baseline; 128 (13%) discontinued use at/after wk 26 (UPA, n=97; ADA, n=31). Baseline demographics and clinical characteristics were broadly similar between pts who continued or discontinued GCs. Median follow-up time after GC discontinuation was 60 wks for UPA and 84 wks for ADA. At the time of GC discontinuation, a numerically higher proportion of pts treated with UPA vs ADA were in disease control (CDAI ≤2.8: 55% vs 32%; CDAI ≤10: 85% vs 68%; DAS28-CRP <2.6: 71% vs 48%; DAS28-CRP ≤3.2: 87% vs 62%) (
Clinical outcomes of pts who discontinued GCs at/after wk 26
| n/N (%) | Pts who discontinued GCs N=128 | |
|---|---|---|
| UPAn=97 | ADAn=31 | |
| CDAI | ||
| ≤10 at discontinuation | 79/93 (85%) | 21/31 (68%) |
| Maintained at 6 months post discontinuation a | 61/66 (92%) | 18/19 (95%) |
| ≤2.8 at withdrawal | 51/93 (55%) | 10/31 (32%) |
| Maintained at 6 months post discontinuation a | 32/43 (74%) | 7/8 (88%) |
| Increase >2 any visit after withdrawal | 1/93 (1%) | 0 |
| DAS28-CRP | ||
| ≤3.2 at withdrawal | 78/90 (87%) | 18/29 (62%) |
| Maintained at 6 months post discontinuation a | 58/64 (91%) | 15/16 (94%) |
| <2.6 at withdrawal | 64/90 (71%) | 14/29 (48%) |
| Maintained at 6 months post discontinuation a | 47/53 (89%) | 11/13 (85%) |
| Increase >0.6 any visit after withdrawal | 6/92 (7%) | 0 |
a As a proportion of pts achieving outcome at GC discontinuation and with observed data 6 months post GC discontinuation.
Conclusion: In pts who achieved disease control and discontinued GCs, disease control was maintained in almost all without worsening disease activity over time following GC discontinuation.
Conclusion: In pts who achieved disease control and discontinued GCs, disease control was maintained in almost all without worsening disease activity over time following GC discontinuation.
REFERENCES:
[1]Smolen JS, et al. Ann Rheum Dis. 2020;79:685–99.
[2]Fleischmann R, et al. Ann Rheum Dis. 2019;78:1454–62.
Acknowledgements: AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, review, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. Medical writing assistance was provided by Julia Zolotarjova, MSc, MWC of AbbVie Inc.
Disclosure of Interests: Roy M. Fleischmann Consultant of: AbbVie, Amgen, BMS, Galvani, Gilead, GSK, Janssen, Eli Lilly, Novartis, Pfizer, and UCB, Grant/research support from: AbbVie, Amgen, Astra-Zeneca, BMS, Flexion, Galvani, Gilead, GSK, Janssen, Eli Lilly, Novartis, Noven, Pfizer, Samumed, Selecta, Teva, UCB, Viela, and Vorso., Bernard Combe Speakers bureau: AbbVie, BMS, Celltrion, Eli Lilly, Gilead-Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche-Chugai, Consultant of: AbbVie, BMS, Celltrion, Eli Lilly, Gilead-Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche-Chugai, Grant/research support from: Pfizer and Roche-Chugai, Andrew Ostor Consultant of: AbbVie, BMS, Eli Lilly, Gilead, Janssen, Novartis, Paradigm, Pfizer, Roche, and UCB., Cesar Francisco Pacheco Tena Consultant of: AbbVie, Astra-Zeneca, Eli Lilly, Gilead, Janssen, Pfizer, Roche, R-Pharm, Sanofi Regeneron, and UCB., Grant/research support from: AbbVie, Astra-Zeneca, Eli Lilly, Gilead, Janssen, Pfizer, Roche, R-Pharm, Sanofi Regeneron, and UCB., Nasser Khan Shareholder of: May own AbbVie stock or stock options, Employee of: AbbVie, Jessica Suboticki Shareholder of: May own AbbVie stock or stock options, Employee of: AbbVie, Anna Shmagel Shareholder of: May own AbbVie stock or stock options, Employee of: AbbVie, Yanna Song Shareholder of: May own AbbVie stock or stock options, Employee of: AbbVie, Ivan Lagunes-Galindo Shareholder of: May own AbbVie stock or stock options, Employee of: AbbVie, Gerd Rüdiger Burmester Speakers bureau: AbbVie, Eli Lilly, Galapagos, Gilead, Janssen, MSD, Pfizer, Roche, Sanofi, and UCB., Consultant of: AbbVie, Eli Lilly, Galapagos, Gilead, Janssen, MSD, Pfizer, Roche, Sanofi, and UCB.