

Background: Up to 30% of RA patients receive monotherapy with biologic (b)DMARDs or JAK inhibitors (i), often due to intolerance of methotrexate (MTX). Monotherapy with IL-6i and JAK-i has been reported to be effective. The EULAR research agenda includes addressing the question of whether tapering of targeted therapy (bDMARDs and JAK-i) used as monotherapy (targeted monotherapy) is possible 1 .
Objectives: To assess if it is feasible to taper (stop or reduce) targeted monotherapy with controlled RA using existing clinical trial data.
Methods: A systematic review of the literature (2014-2021), cited in Medline, Embase and the Cochrane Library, was performed. Meta-analyses were conducted using random effects models. Forest and funnel plots were created and heterogeneity calculated.
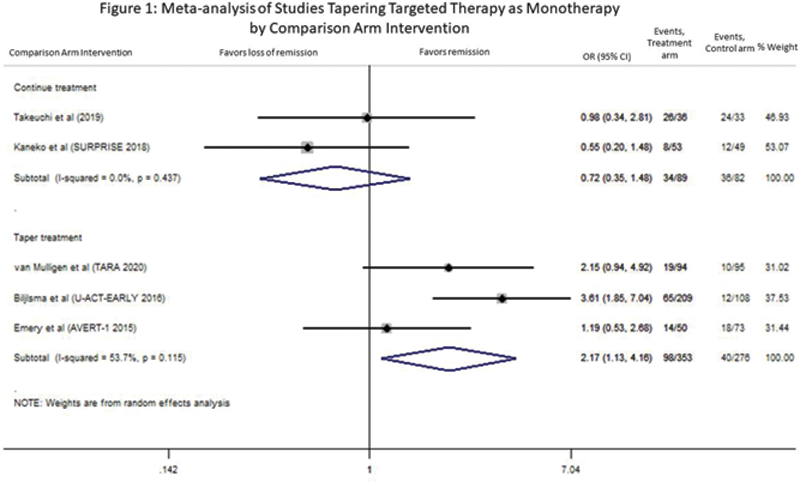
Results: Our search yielded 5762 citations. After de-duplication, screening of titles/abstracts and review of full text articles, we identified 5 studies comparing tapering of targeted monotherapy (TNF-i, tocilizumab (TCZ), abatacept (ABA) and baricitinib) to continuing therapy or other tapering regimens (
Included studies.
| Author/year | n | Early RA | Mean Age Range | Baseline | Tapering strategy | Comparison arm intervention | Remission Outcome | Follow up |
|---|---|---|---|---|---|---|---|---|
| treatment | ||||||||
| van Mulligen 2020 | 189 | No | 56-57 | csDMARD + TNFi | Taper csDMARD then TNFi | Taper in reverse order | DAS44 < 1.6 | 24 mos |
| Kaneko | 102 | No | 54-58 | TCZ+MTX | Stop TCZ | Continue MTX | DAS28 < 2.6 | 104 wks |
| 2018 | vs TCZ | |||||||
| Bijlsma | 299 | Yes | 54 | TCZ+MTX | Gradual taper MTX 1 st then TCZ | Gradual taper MTX | DAS28 < 2.6+SJC≤4 | 104 wks |
| 2016 | vs TCZ | |||||||
| vs MTX | ||||||||
| Emery | 176 | Yes | 45-49 | ABA+MTX | Stop ABA | Stop ABA Taper MTX off | DAS28-CRP<2.6 | 18 mos |
| 2015 | vs ABA | |||||||
| vs MTX | ||||||||
| Takeuchi | 69 | Yes | 48-53 | Bari 4mg | Reduce 2mg | Continue 4mg | CDAI < 2.8 | 48 wks |
| 2019 |
ABA abatacept, Bari baricitinib, CDAI Clinical disease activity index, csDMARDS conventional synthetic DMARDs, DAS28 Disease Activity Score 28, MTX methotrexate, SJC swollen joint count, TCZ tocilizumab, wks weeks, mos months.
Conclusion: There are no trials designed to compare tapering targeted monotherapy to continuing it, indicating a significant gap in knowledge in an area of increasing clinical relevance for our patients. There was insufficient evidence to demonstrate the significant effects of tapering targeted monotherapy in RA. Only one study out of 5 compared stopping targeted monotherapy to continuing therapy (MTX), and reported a low OR of remission. Three studies tapered therapy in both arms and one study performed a dose reduction. Our review suggests that stopping targeted monotherapy is unlikely to maintain disease control. More gradual tapering schemes, dose reduction and early treatment of disease may be associated with more successful tapering. More studies are needed to better inform our patients. Currently, we do not recommend stopping targeted monotherapy in RA.
REFERENCES:
[1]Smolen JS, Landewé RBM, Bijlsma JWJ, et al.Ann Rheum Dis 2020;79:685-99.
Disclosure of Interests: Charis Meng: None declared, Diviya Rajesh: None declared, Deanna Jannat-Khah Shareholder of: AstraZeneca, Cytodyn, Walgreens, Omar Bruce: None declared, Bridget Jivanelli: None declared, Vivian Bykerk Consultant of: Amgen, Bristol Myers Squibb, Genzyme, Gilead, Janssen, Pfizer, Sanofi-Aventis, UCB, Grant/research support from: NIH (NIAID/NIAMS) grant 1UH2AR067691-01 GRANT11652401 and The Cedar Hill Foundation; institution received grants from Bristol Myers Squibb and Amgen