

Background: The primary treatment target for patients with active rheumatoid arthritis (RA) is sustained clinical remission (REM) or low disease activity (LDA). 1,2 A greater proportion of patients with RA and inadequate response to methotrexate (MTX) receiving the JAK inhibitor, upadacitinib (UPA), achieved REM/LDA compared with adalimumab (ADA), both with background MTX, through 26 weeks in the phase 3, SELECT-COMPARE trial. 3
Objectives: We assessed sustainability of response over 3 years in UPA-treated patients with RA.
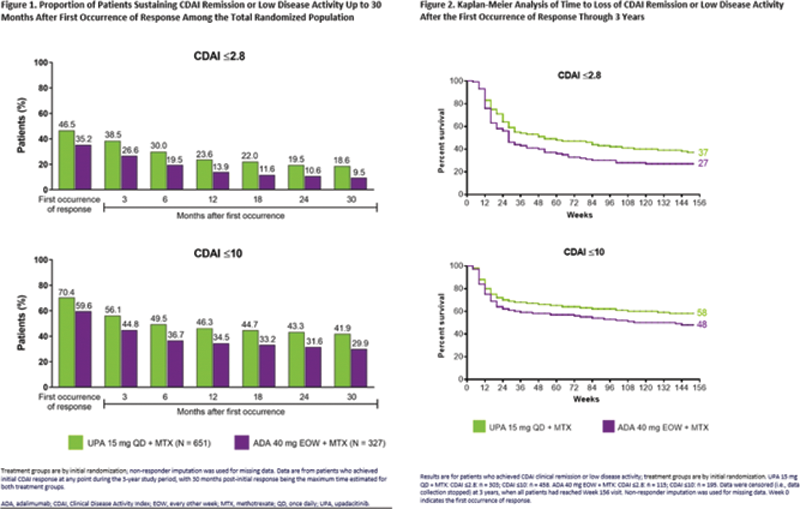
Methods: SELECT-COMPARE included a 26-week, double-blind, placebo (PBO)-controlled period, a 48-week, double-blind active comparator-controlled period, and an ongoing long-term extension for up to 10 years. Patients on background MTX received UPA 15 mg once daily, PBO, or ADA 40 mg every other week. Patients who did not achieve at least 20% improvements in tender and swollen joint counts (Weeks 14-22) or LDA (CDAI ≤10 at Week 26) were rescued from UPA to ADA or PBO/ADA to UPA. This post hoc analysis evaluated clinical REM (CDAI ≤2.8; SDAI ≤3.3), LDA (CDAI ≤10; SDAI ≤11), and DAS28(CRP) <2.6/≤3.2 at first occurrence (prior to treatment switch [rescue]), as well as over 3 years following initial response in patients randomized to UPA or ADA. For those patients who achieved REM/LDA, Kaplan-Meier was used to define the time from when the response was first achieved to the earliest date at which the response was lost at two consecutive visits, discontinuation of study drug, or losing response at the time of rescue. The predictive ability of time to CDAI REM/LDA was assessed using Harrell’s concordance (c)-index (range: 0 [all predictions wrong] to 1.0 [perfect predictive ability]. Non-responder imputation was used for missing data.
Results: Through 3 years, a significantly higher proportion of patients receiving UPA + MTX vs ADA + MTX achieved CDAI REM (47% vs 35%,
P
= 0.001) as well as CDAI LDA (70% vs 60%,
P
= 0.001). At 30 months after first occurrence of response, CDAI REM/LDA was sustained in 19%/42% of patients randomized to UPA and 10%/30% of patients randomized to ADA (
Conclusion: Among patients with inadequate response to MTX, a higher proportion receiving UPA + MTX achieved remission or LDA across disease activity measures vs ADA + MTX. UPA-treated patients demonstrated a consistently higher sustained response rate over 3 years compared to those receiving ADA. Furthermore, significant proportions of patients who lost response on either UPA or ADA were able to recapture remission or LDA.
REFERENCES:
[1]Smolen et al. Ann Rheum Dis 2020;79:685–99.
[2]Singh et al. Arthritis Rheumatol 2016;68:1–26.
[3]Fleischmann et al. Arthritis Rheumatol 2019;71:1788–1800.
Acknowledgements: AbbVie funded these studies and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. No honoraria or payments were made for authorship. Medical writing support was provided by Matthew Eckwahl, PhD, of AbbVie.
Disclosure of Interests: Peter Nash Speakers bureau: AbbVie, BMS, Pfizer, Gilead/Galapagos, Sanofi, Celgene, Novartis, Lilly, Janssen, UCB, Samsung, MSD, Roche, Consultant of: AbbVie, BMS, Pfizer, Gilead/Galapagos, Sanofi, Celgene, Novartis, Lilly, Janssen, UCB, Samsung, MSD, Roche, Grant/research support from: AbbVie, BMS, Pfizer, Gilead/Galapagos, Sanofi, Celgene, Novartis, Lilly, Janssen, UCB, Samsung, MSD, Roche, Arthur Kavanaugh Consultant of: AbbVie Inc., Amgen, Astra-Zeneca, BMS, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB, Grant/research support from: AbbVie Inc., Amgen, Astra-Zeneca, BMS, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB, Maya H Buch Speakers bureau: AbbVie, Boehringer Ingleheim, Eli Lilly, Merck-Serono, and Sanofi, Consultant of: AbbVie, Boehringer Ingleheim, Eli Lilly, Merck-Serono, and Sanofi, Grant/research support from: Pfizer, Gilead, and UCB, Bernard Combe Consultant of: AbbVie, BMS, Celltrion, Gilead, Galapagos, Janssen, Eli Lilly, MSD, Pfizer, Roche Chugai, Louis Bessette Speakers bureau: Amgen, BMS, Janssen, Roche, UCB, AbbVie, Pfizer, Merck, Celgene, Sanofi, Eli Lilly, Novartis, Sandoz, Gilead, Fresenius Kabi, and Teva, Consultant of: Amgen, BMS, Janssen, Roche, UCB, AbbVie, Pfizer, Merck, Celgene, Sanofi, Eli Lilly, Novartis, Sandoz, Gilead, Fresenius Kabi, and Teva, Grant/research support from: Amgen, BMS, Janssen, Roche, UCB, AbbVie, Pfizer, Merck, Celgene, Sanofi, Eli Lilly, Novartis, Sandoz, Gilead, Fresenius Kabi, and Teva, In-Ho Song Shareholder of: AbbVie Inc., Employee of: Amgen, BMS, Janssen, Roche, UCB, AbbVie, Pfizer, Merck, Celgene, Sanofi, Eli Lilly, Novartis, Sandoz, Gilead, Fresenius Kabi, and Teva, Tim Shaw Shareholder of: AbbVie Inc., Employee of: AbbVie Inc., Yanna Song Shareholder of: AbbVie Inc., Employee of: AbbVie Inc., Jessica Suboticki Shareholder of: AbbVie Inc., Employee of: AbbVie Inc., Roy M. Fleischmann Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, GSK, Janssen, Novartis, Pfizer Inc, Sanofi-Aventis, and UCB, Grant/research support from: AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Genentech, Janssen, Novartis, Pfizer Inc, Regeneron, Roche, Sanofi-Aventis and UCB